
Qualified surgeons may offer laparoscopic distal gastrectomy as an alternative to open distal gastrectomy among those with clinical T4a gastric cancer.

Your AI-Trained Oncology Knowledge Connection!


Qualified surgeons may offer laparoscopic distal gastrectomy as an alternative to open distal gastrectomy among those with clinical T4a gastric cancer.

Co-hosts Kristie L. Kahl and Andrew Svonavec highlight what to look forward to at the 67th Annual ASH Meeting in Orlando.

In a spotlight session at the Chemotherapy Foundation Symposium, Anne Chiang, MD, PhD, covered developments in the field of LS-SCLC.
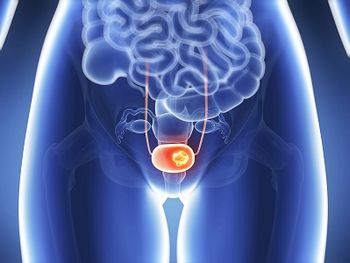
During treatment with TARA-002, there were no grade 3 or higher TRAEs, and TRAEs did not lead to any treatment discontinuations in patients with BCG-naïve NMIBC.

Data from the BRUIN-CLL-321 trial led to the FDA granting traditional approval to pirtobrutinib in CLL/SLL indications.

A 6-month CR rate of 62% was observed with detalimogene voraplasmid in treating patients with BCG-unresponsive non–muscle invasive bladder cancer.

AI revolutionizes palliative oncology by enhancing prognostication, symptom management, and personalized care for patients with hematologic malignancies.

Based on a patient’s SCLC subtype, and Schlafen 11 status, patients will be randomly assigned to receive durvalumab alone or with a targeted therapy in the S2409 PRISM trial.

Phase 1 data related to CLN-049 for patients with acute myeloid leukemia will be presented at the 2025 ASH Annual Meeting and Exposition.

Daniel Peters, MD, aims to reduce the toxicity associated with AML treatments while also improving therapeutic outcomes.

Data from a prospective study showed that the IsoPSA prostate cancer test outperformed total PSA and free PSA in men 50 years or older with elevated PSA level.

Results from the phase 1b ENGAGER-PSMA-01 trial showed deep PSA reductions and a favorable CRS profile among patients with taxane-naive CRPC.

Findings from numerous clinical trials vindicating the addition of immunotherapy to first-line chemotherapy in SCLC have emerged over the past several years.

Rising cancer diagnoses in the US highlight urgent needs for improved oncology education, workforce distribution, and care infrastructure, especially in rural areas.

Patients with AML will experience different toxicities based on the treatment they receive, whether it is intensive chemotherapy or targeted therapy.

Findings from the HERIZON-BTC-01 trial support HER2 as a valid therapeutic target in biliary tract cancer.

Edward Chu, MD, a member of the gastrointestinal editorial advisory board, died of glioblastoma in November.
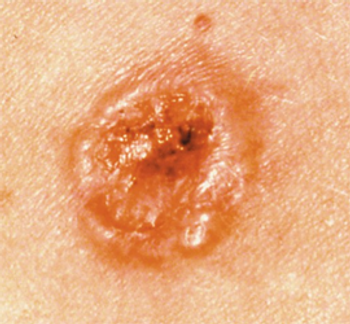
Updated results from the phase 1 CK-301-101 trial support the updated label for cosibelimab in this cutaneous squamous cell carcinoma population.

A younger patient with AML who is more fit may be eligible for different treatments than an older patient with chronic medical conditions.

Experts from Georgia Cancer Center highlight ongoing retrospective studies, translational research, and other initiatives across different cancers.

The developers plan to submit a new drug application to regulatory authorities for JS001sc for the treatment of first-line nonsquamous NSCLC.

According to Daniel Peters, MD, the recent FDA approvals of revumenib and ziftomenib in AML are some of the most exciting developments in the field.

Breast cancer care providers make it a goal to manage the adverse effects that patients with breast cancer experience to minimize the burden of treatment.

According to Rachel Greenup, MD, MPH, some of the barriers to getting optimal treatment for breast cancer include access, insurance, and baseline understanding or knowledge.

HRQOL scores were similar among patients who received a radical cystectomy or bladder persevering therapy for non-muscle invasive bladder cancer.

Thomas Hope, MD, asserts that legislation aimed at resolving infiltration-related AEs does not address any relevant clinical issue.

Social workers and case managers may have access to institutional- or hospital-level grants that can reduce financial toxicity for patients undergoing cancer therapy.

Genetic backgrounds and ancestry may hold clues for better understanding pancreatic cancer, which may subsequently mitigate different disparities.

A recent study found that the number of practicing oncologists is declining as the US population ages and cancer diagnoses continue to increase.

Investigators noted 1 instance of rectovaginal fistula in the PEG gel arm, although rapid postoperative recovery occurred.