
Experts met to debate recently presented trials in the hematologic oncology after the 2023 American Society of Clinical Oncology Annual Meeting.

Your AI-Trained Oncology Knowledge Connection!


Experts met to debate recently presented trials in the hematologic oncology after the 2023 American Society of Clinical Oncology Annual Meeting.

The regulatory decision to approve elacestrant in European patients with estrogen receptor–positive, HER2-negative locally advanced or metastatic breast cancer and an activating ESR1 mutation was based on data from the phase 3 EMERALD study.

Jennifer L. Garson, PA-C, gave an overview of follicular lymphoma and how she manages adverse effects in her institution.

Findings from a cross-sectional study support monitoring puberal development to estimate probabilities of future fertility among male childhood cancer survivors.
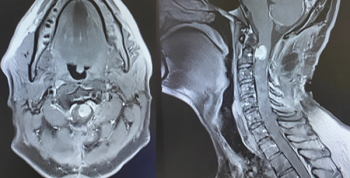
A rare case of spinal atypical teratoid/rhabdoid tumor is presented in an adult man after presenting with neck pain and bilateral upper extremity paralysis.
Data from the phase 3 ASCERTAIN trial support the European Commission’s approval of oral decitabine and cedazuridine as a treatment for those with newly diagnosed acute myeloid leukemia.
Findings from a meta-analysis indicate that interventions for caregivers of patients with advanced cancer may improve self-efficacy and grief.

Combining regorafenib with nivolumab and chemotherapy appears to improve progression free survival at 6 months among those with advanced esophagogastric adenocarcinoma.
Several discrepancies in care must be considered to properly address psychological needs in patients with lymphoma, including patient/doctor communication, information provision, and applying distress guidelines.

Findings from the phase 3 KEYNOTE-A18 trial support the supplemental biologics license application for a pembrolizumab-based regimen as a treatment for patients with newly diagnosed, high-risk, locally advanced cervical cancer.

Arvind N. Dasari, MD, and Cathy Eng, MD, gave an overview on the use of fruquintinib in colorectal cancer.

Adding all-trans retinoic acid to non-intensive chemotherapy appears to produce higher toxicity in elderly unfit patients with acute myeloid leukemia harboring NPM1 mutations.

Data from the phase 3 LITESPARK-005 trial support the supplemental new drug application for belzutifan as a treatment for patients with advanced renal cell carcinoma.

KT-333 is under investigation as a treatment for adult patients with relapsed/refractory lymphomas, leukemias, and solid tumors in a phase 1 clinical trial.
Objective response rate appears to be numerically higher for patients receiving zanubrutinib for relapsed/refractory mantle cell lymphoma in the second-line setting than in later lines of treatment.

Investigators of the phase 1b/2 KontRASt-01 trial will continue their evaluation of TNO155 plus JDQ433 in KRAS G12C-mutated solid tumors as part of an expansion portion of the trial.

Thomas G. Martin, MD, discussed with his colleagues cytokine release syndrome after treatment with teclistamab for patients with multiple myeloma.

Findings from the phase 2 ALYCANTE trial support axicabtagene ciloleucel as a second-line treatment for patients with relapsed/refractory large B-cell lymphoma who are ineligible to undergo a transplant.

Data from a previous MPN LANDMARK survey highlight that many patients with polycythemia vera experience symptoms that lead to reduced quality of life.

Responses in patients with hormone receptor–positive, HER2-mutated breast cancer who crossed over to receive neratinib plus fulvestrant and trastuzumab support the necessity of neratinib in the triplet regimen.

Pharmaceutical, gynecologic oncology, and physician perspectives shed light on potentially mitigating the ongoing carboplatin and cisplatin shortages’ effects on cancer care in the United States.

Treatment with zongertinib produces low rates of EGFR-mediated adverse effects among patients with HER2-mutated non–small cell lung cancer in the phase 1a/b BEAMION Lung-1 trial.

Robert Motzer, MD, led an expert panel to discuss the phase 3 CONTACT-03 which examined patients with renal cell carcinoma.

Baseline characteristics do not appear to correlate with long-term benefits for patients receiving durvalumab plus tremelimumab and chemotherapy for metastatic non–small cell lung cancer in the phase 3 POSEIDON trial.

Ryan Jacobs, MD, and Alan Pierre Zausner Skarbnik, MD, discussed the use of acalabrutinib and zanubrutinib in patients with relapsed/refractory chronic lymphocytic leukemia.

With the approval of momelotinib by the FDA, the updated use for its role in myelofibrosis will potentially change clinical practice, according to Ruben Mesa, MD.

Patients with myelofibrosis and anemia can now receive treatment with momelotinib following the agent’s approval by the FDA based on results from the phase 3 MOMENTUM trial.
Investigators report no differences in progression-free survival among patients with nonseminomatous testicular cancer who received surveillance or adjuvant chemotherapy in a cohort study.

Findings from the phase 3 AEGEAN trial suggest that patients with EGFR-mutated non–small cell lung cancer experience similar outcomes whether receiving durvalumab or placebo.

Data from the phase 2 JACKPOT8 PARTB study support the new drug application for golidocitinib as a treatment for relapsed/refractory peripheral T-cell lymphoma in China.