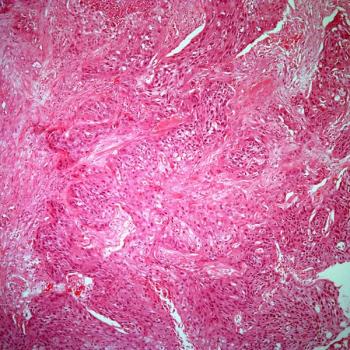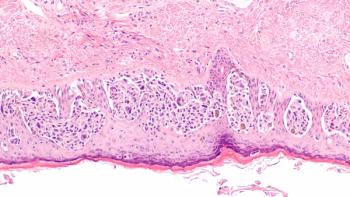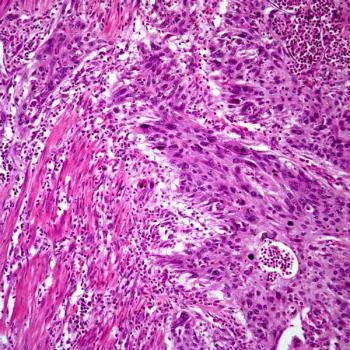
The results of the phase 3 LUNAR trial evaluating tumor treating fields plus standard-of-care therapies among patients with metastatic non–small cell lung cancer are “encouraging,” according to an expert from Winship Cancer Institute of Emory University.