
Compared with a control therapy, ruxolitinib increased overall response and improved failure-free survival for patients with glucocorticoid-refractory or -dependent chronic graft-versus-host disease.

Your AI-Trained Oncology Knowledge Connection!


Compared with a control therapy, ruxolitinib increased overall response and improved failure-free survival for patients with glucocorticoid-refractory or -dependent chronic graft-versus-host disease.
The combination of venetoclax and azacitidine will be considered as a treatment for patients with newly diagnosed, higher-risk myelodysplastic syndrome.

Treatment with trastuzumab, pertuzumab, and docetaxel appears to yield similar efficacy to trastuzumab emtansine in patients with ErbB2-positive breast cancer.

Robert A. Figlin, MD, spoke with CancerNetwork® about emerging second-line therapies as the paradigm of first-line treatment evolves for patients with metastatic clear cell renal cell carcinoma.
Early-phase data indicate that loncastuximab tesirine-lpyl elicited promising, long-lasting responses in patients with mantle cell lymphoma.
Data from a first-of-its-kind study indicated that unnecessary use of antibiotics may lead to early-onset colon cancer.

The combination of trilaciclib plus chemotherapy has been granted a fast track designation by the FDA for the treatment of locally advanced metastatic triple-negative breast cancer.

CancerNetwork® sat down with Edmund Qiao at the 2021 American Society of Clinical Oncology Annual Meeting to talk about prostate-specific antigen screening and prostate cancer prevention in African American men.
Take a look at some of the important updates from CancerNetwork last week you might have missed in the world of oncology from the FDA and the journal ONCOLOGY.

This special edition of the “Oncology Peer Review On-The-Go” dives into recent updates and important treatment strategies for extensive-stage SCLC management.
Patients with metastatic triple-negative breast cancer being treated with leronlimab and carboplatin have experienced a decrease in cancer-associated macrophage-like cells and an increase in survival benefit.
Investigators will continue treating patients with GEN-1 plus neoadjuvant chemotherapy following the results of a pre-planned interim safety review of the phase 1/2 OVATION 2 study.

The phase 2 GIMEMA LAL1913 trial identified poor outcomes and increased minimal residual disease persistence among patients with Philadelphia-like acute lymphoblastic leukemia.

At ASCO 2021, Stephen Liu, MD, discussed his excitement for results of the IMpower010 trial of atezolizumab in patients with early-stage resected non–small cell lung cancer
Results from the phase 3 COSMIC-311 trial indicated that cabozantinib elicited a promising survival benefit in patients with radioiodine-refractory differentiated thyroid cancer.
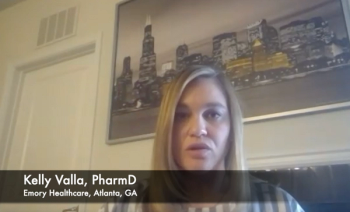
Tazemetostat use only requires dosing modifications in a small percentage of patients, according to an oncology pharmacist.

Pembrolizumab plus chemotherapy as adjuvant/neoadjuvant therapy demonstrated positive even-free survival outcomes in the KEYNOTE-522 study for patients with triple-negative breast cancer.

Eric J. Sherman, MD, highlights several drugs that are being used to treat RET-positive thyroid cancer.

Eric J. Sherman, MD, examines the phase 3 COSMIC-311 trial with cabozantinib in radioiodine-refractory differentiated thyroid cancer.

The ROCK-2–targeting agent belumosudil is now approved by the FDA to treat adult and pediatric patients with chronic graft-versus-host disease after 2 prior lines of therapy.

Foundation Medicine announced the expanded indication for FoundationOne Liquid CDx as a companion diagnostic to identify MET exon 14 skipping mutations in metastatic non–small cell lung cancer.
Findings from the OCTOPUS Consortium of trial data indicated that weight-based chemotherapy dosing may improve outcomes for obese patients with colorectal cancer.

CancerNetwork® sat down with Richard D. Kim, MD, of the Moffitt Cancer Center at the 2021 ASCO Annual Meeting to talk about the many ways oncologists can approach treating hepatocellular carcinoma with combination regimens.

Moreau discussed conclusions drawn from part 2 of the CASSIOPEIA trial and the need for further follow-up with bortezomib, thalidomide and dexamethasone plus maintenance daratumumab in newly diagnosed multiple myeloma.

Men diagnosed with mCPRC who are treated with 117Lu-PSMA may be assessed using nomograms to help predict outcomes.
Data in patients with colorectal cancer indicates that the Signatera MRD test may help guide treatment decisions in patients with resectable disease.

Adverse effects in patients taking tazemetostat were manageable, with most patients reporting low-grade fatigue, pain, and nausea.

A new study has adapted CAR to look more human to the body, which will then in turn yield longer remission rates for pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia.

A new phase 2 study shows that patients who were treated with HIPEC and Carboplatin, did not have better progression-free or overall survival.
Adjuvant radiation therapy, when compared with early salvage radiation therapy, reduced the risk of all-cause mortality for patients with adverse pathology at radical prostatectomy.