Glofitamab with polatuzumab vedotin benefited patients with high-grade B-cell lymphomas and who failed prior CAR T-cell therapy.

Your AI-Trained Oncology Knowledge Connection!


Glofitamab with polatuzumab vedotin benefited patients with high-grade B-cell lymphomas and who failed prior CAR T-cell therapy.

Phase 2 data support epcoritamab monotherapy as a promising chemotherapy-free option for older patients with newly diagnosed LBCL.
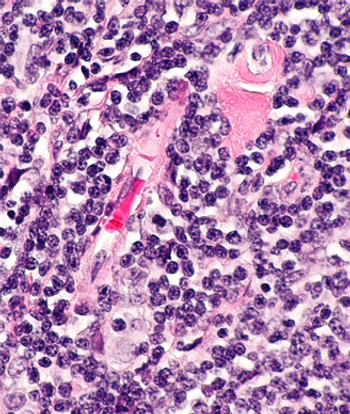
Findings from the phase 1/2 CaDAnCe-101 trial showed that promising ORRs in R/R WM and R/R CLL/SLL stemmed from BGB-16673 treatment.

In patients with lens-refractory multiple myeloma, cilta-cel generated deeper minimal residual disease rates across all patient subgroups.

Pelabresib and ruxolitinib combination maintains consistent reduction of symptoms and anemia in patients with myelofibrosis.

For patients with CLL and SLL, sonrotoclax with zanubrutinib yielded high rates of overall response in the phase 1/1b BGB-11417-101 study.

In patients with VEXAS Syndrome with or without MDS, ESAs/luspatercept generated positive hematologic improvement-erythroid responses.

A panel of cancer survivorship experts outline major areas of focus and care models for improving outcomes among pediatric and adult survivors of cancer.

Patients with newly diagnosed multiple myeloma experienced sustained MRD negativity with isatuximab/bortezomib/lenalidomide/dexamethasone.

Findings from DREAMM-7 support belantamab mafodotin plus bortezomib and dexamethasone as a standard of care in relapsed/refractory multiple myeloma.

Samatha Shenoy, NP, MSN, highlighted lifestyle recommendations to help patients who are receiving talquetamab treatment for multiple myeloma.

The 2 main pafolacianine components, a folate analog and a dye, are commonly used in other medical applications.

Atrial fibrillation occur at a smaller likelihood with second-generation BTK inhibitors vs first-generation BTK inhibitors in B-cell hematologic cancers.

Phase 3 data show meaningful benefits with uproleselan in patients with primary refractory AML and post-transplant survival.

Zanubrutinib maintained a PFS benefit at 5 years vs benadmustine and rituximab for patients with CLL/SLL.
Tisagenlecleucel shows high rates of MRD-negative status among patients with relapsed/refractory follicular lymphoma in the ELARA trial.

The venetoclax regimen improved CR/CRi rates for patients with acute myeloid leukemia and myelodysplastic syndromes.

For patients with acute lymphoblastic leukemia and mantle cell leukemia, lymphodepletion then brexu-cel show positive efficacy and safety outcomes.

Minimal tumor lysis syndrome was observed with venetoclax combined with hypomethylating agents in patients with myelodysplastic syndrome.

Using bispecific antibodies before or after CAR T-cell therapy in multiple myeloma is an area of education for community oncologists.

The IPSS-del(5q) Scoring System shows that factors including male sex, cytopenias, and complex genetic background worsen the prognosis of MDS-del(5q).

Navtemadlin shows influence in myelofibrosis hallmarks such as CD34-positive cell proliferation and pro-inflammatory cytokines in the phase 3 BOREAS trial.
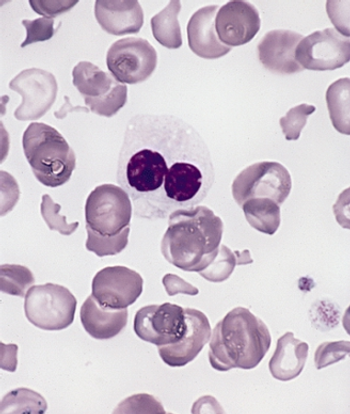
For patients with MDS who are relapsed/refractory to erythropoietin stimulating agents, low-dose deferasirox demonstrated promising efficacy and tolerability outcomes.

Andrew M. Evens, DO, MBA, MSc, discusses the importance of meeting the emotional and physical needs of cancer survivors to help them lead fruitful lives.

Almost a 100% complete response rate was noted with Zilovertamab Vedotin/R-CHP for patients with DLBCL.

All evaluable patients achieved minimal residual disease negativity following teclistamab-based treatment in the phase 3 MajesTEC-4/EMN30 trial.

Acalabrutinib-based treatment also improves overall survival vs standard chemoimmunotherapy in the phase 3 AMPLIFY trial.

PFS and OS were significantly improved with subcutaneous daratumumab vs active monitoring in patients with smoldering multiple myeloma.
For patients with relapsed/refractory large B-cell lymphoma, PFS and OS data from CD-19-directed, 4-1BB CAR T-cell Liso-cel therapy were consistent with prior results.

Data from the phase 3 ASC4FIRST trial support asciminib as a standard of care in newly diagnosed chronic myeloid leukemia in chronic phase.