
Amid the recent cisplatin shortages across the United States, a pharmacy expert discusses how the treatment of bladder cancer has been affected, especially in the muscle-invasive subtype.

Your AI-Trained Oncology Knowledge Connection!


Ariana Pelosci, managing editor for CancerNetwork® and the journal ONCOLOGY®, has been with the team since June 2021. She specializes in both web and print, and runs the social media accounts for CancerNetwork®.
She graduated from the University of Delaware, where she studied Media Communications and minored in journalism and marketing. At heart, she is a Jersey girl, and you can always find her down the shore during her free time.
Ariana loves to read, specifically historical or contemporary fiction. Follow Ariana on Twitter @APelosci or email her at apelosci@mjhlifesciences.com.

Amid the recent cisplatin shortages across the United States, a pharmacy expert discusses how the treatment of bladder cancer has been affected, especially in the muscle-invasive subtype.

Patients with relapsed/refractory multiple myeloma may now receive talquetamab following the agent’s accelerated approval by the FDA.

Patients with metastatic RET fusion-positive non–small cell lung cancer may now be treated with pralsetinib, which has been granted regular approval by the FDA.

Results from the phase 3 SUNLIGHT trial support the approval of trifluridine/tipiracil plus bevacizumab in patients with previously treated, metastatic colorectal cancer.

Experts in the genitourinary and gynecology fields discuss how they have adapted and adjusted treatment for their patients amid the United States’ shortage of cisplatin and carboplatin.
Sandra P. D’Angelo, MD, reviews current treatments in sarcoma, and how she interacts with her multidisciplinary counterparts to offer the best care to her patients.

Results from the phase 1/2 NP30179 trial led the European Commission to grant conditional marketing authorization to patients with relapsed/refractory diffuse large B-cell lymphoma.

Blinatumomab has been granted full approval by the FDA for patients with B-cell acute lymphoblastic leukemia and minimal residual disease of 0.01% or more.

Results from the phase 1/2 NP30179 analyzing glofitamab in patients with relapsed/refractory diffuse large B-cell lymphoma and large B-cell lymphoma are reported to have helped lead to its approval.

Results from the phase 2 DARIA trial found an improved overall response rate in patients given daratumumab, ixazomib, and dexamethasone with relapsed/refractory multiple myeloma who were previously treated with lenalidomide.

Treatment with patritumab deruxtecan appears to yield improved responses in patients who have been heavily pretreated for metastatic breast cancer.
Results from the phase 2b HERIZON-BTC-01 trial show positive efficacy and safety responses in patients with locally advanced, unresectable or metastatic biliary tract cancer.

Patients with BRCA-mutated metastatic castration-resistant prostate cancer can now receive treatment with olaparib plus abiraterone/prednisone or prednisolone following the combination’s approval by the FDA.

18F-rhPSMA-7.3 injection is now available to help identify PSMA-positive lesions during PET imaging for patients with metastatic or recurrent prostate cancer.

Ashley E. Rosko, MD, reviews unmet needs and treatment sequencing for patients with multiple myeloma.
A post hoc analysis indicates continuous intravenous infusion of doxorubicin does not yield additional benefit for patients with soft-tissue sarcoma compared with bolus delivery methods.

Findings from the phase 2 MAJIC-PV trial highlight a superior event-free survival and overall survival following ruxolitinib vs best available treatment in patients with polycythemia vera who were intolerant/resistant to hydroxycarbamide.

Treatment with 20 mg/m2 of lenalidomide in patients under 22 years old who have pilocytic astrocytomas and optic pathway gliomas appears to be safe and effective.

Investigators say that among those with platinum-refractory recurrent or metastatic head and neck squamous cell carcinoma who responded to nivolumab plus ipilimumab duration of response was not reached.

Pharmacy director Kirollos Hanna, PharmD, BCPS, BCOP, FACC, discusses how to navigate shared toxicities between combination immunotherapy and VEGF inhibitors for patients with renal cell carcinoma.
During the ODAC meeting, members voted to restrict the use of olaparib plus abiraterone and prednisone or prednisolone to patients with BRCA-mutated metastatic castration-resistant prostate cancer.
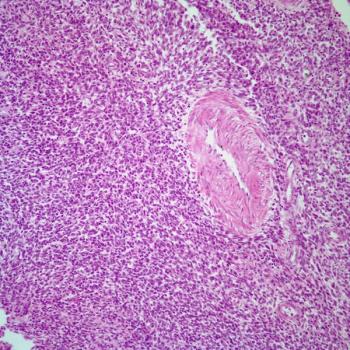
The European Medicines Agency validates the potential indication of dostarlimab/chemotherapy in mismatch repair deficient/microsatellite instability–high endometrial cancer based on the phase 3 RUBY trial.

Investigators cite the need for further study of the 25 mg, 3 times weekly exemestane dosing schedule in prevention studies among those with post-menopausal, stage 0 to II estrogen receptor–positive breast cancer who can’t tolerate daily adjuvant treatment.

A senior physician’s assistant from Johns Hopkins examines why adverse effect management is so crucial and reviews how to help maintain quality of life while undergoing treatment.

Experts in the multiple myeloma space review available therapies such as CAR T-cell therapy, bispecifics, and B-cell maturation antigen–targeted agents for patients with multiple myeloma.

Results from cohort K of the phase 1b/2 KEYNOTE-869 trial led to the accelerated approval of enfortumab vedotin plus pembrolizumab in patients with locally advanced or metastatic urothelial cancer who cannot receive cisplatin-based chemotherapy.

Based on results from the phase 2 KEYNOTE-158, KEYNOTE-164, and KEYNOTE-051 trials, the FDA has given full approval to pembrolizumab for patients with microsatellite instability-high or mismatch repair deficient solid tumors.
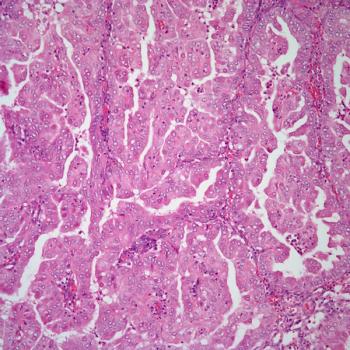
The phase 3 RUBY trial of dostarlimab plus carboplatin and paclitaxel significantly improved progression-free survival in patients with dMMR/MSI-H or MMRp/MSS endometrial cancer.

In the SOLAR phase 1b trial, a clinical benefit was observed when patients with RAS-mutated ovarian or endometrial cancer were given olaparib plus selumetinib.

Results from the phase 2 CDRB436G2201 trial lead to the approval of dabrafenib plus trametinib in patients with low-grade glioma and a BRAF V600E mutation who require systemic therapy.