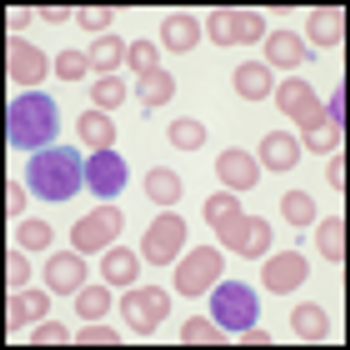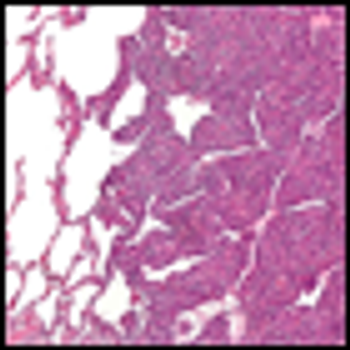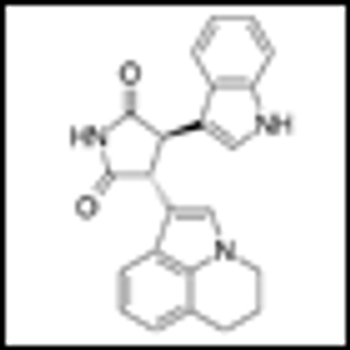
Ponatinib showed significant antileukemic activity in patients with CML and ALL, according to a phase II study that included a wide range of disease stages and mutation status; patients in the trial had a relatively high rate of adverse thrombotic events, an issue which has led to recent regulatory controversy surrounding the drug.