
Professor of pharmacology Eric Winer, MD, spoke about a publication he authored exploring the state of oncologist burnout and how it impacts practice.

Your AI-Trained Oncology Knowledge Connection!


Professor of pharmacology Eric Winer, MD, spoke about a publication he authored exploring the state of oncologist burnout and how it impacts practice.

Workplace burnout in medicine and oncology can lead to stress, exhaustion, fatigue, and a lack of interest in work, according to Eric P. Winer, MD.

Eric P. Winer, MD, said, “[Health care] has a workforce problem, which is part of the reason why we need to embrace nurse practitioners and PAs or APPs.”

“Although telehealth is a great thing when used properly, telehealth also puts a barrier between the doctor and the patient,” said Eric P. Winer, MD.
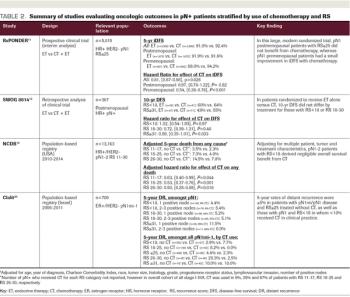
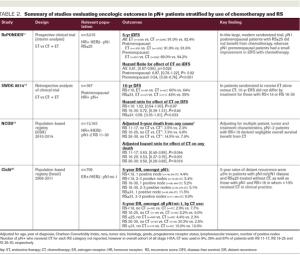
ABSTRACT The 21-gene Recurrence Score (RS) assay has been validated as both a prognostic and predictive tool in node-negative (pN0), estrogen receptor–positive (ER+), HER2-negative (HER2–) breast cancer. A large body of evidence supports the clinical utility of the RS in the node positive (pN+) population as well. Retrospective analyses of archived tissue from multiple clinical trials have found the RS to be prognostic in both endocrine therapy (ET)-treated and chemotherapy-treated patients with pN+ disease. Distribution of RS results in pN+ patients have also been consistent with those of pN0 populations. Data from the SWOG 8814 trial and large population-based registries further support the prognostic and potential predictive value of the RS. Specifically, patients with 1 to 3 positive nodes and RS less than 18 derived negligible benefit from adjuvant chemotherapy in these studies. In the prospective West German Study Group PlanB and ADAPT trials, pN+ patients with RS less than 11 and RS ≤25, respectively, who were treated with ET alone experienced excellent outcomes. Finally, 5-year results of the RxPONDER clinical trial randomizing patients with 1 to 3 positive nodes and RS ≤25 to ET alone vs ET plus chemotherapy confirmed an absence of chemotherapy benefit in postmenopausal patients. Clinical practice guidelines support use of the RS in the pN+, ER+/HER2– population, and many institutions have adopted the RS to guide clinical decision-making, resulting in a net reduction of adjuvant chemotherapy use. This review highlights the existing data supporting the prognostic and predictive ability of the RS in pN+ disease, current practice patterns related to RS use in this population, and emerging applications.

We acknowledge that the “more is better” approach may not always hold true. For example, preclinical data provided a rationale for combining pertuzumab with T-DM1, but recent reports suggest that this strategy may not prove more effective than single-agent T-DM1 therapy in the clinic.

In this article, we describe the long natural history of HR+ breast cancer and review current research and clinical strategies to address this clinical challenge.

The majority of invasive breast cancer patients present with hormone receptor-positive disease, and modulation of estrogen receptor (ER) activation is an essential component of systemic adjuvant therapy for these women. While tamoxifen has traditionally been the primary adjuvant endocrine therapy for all ER-positive women, recent trials evaluating the use of aromatase inhibitors (AIs) have challenged this standard in postmenopausal women, and ongoing trials are examining the optimal use of endocrine therapy in younger women. Issues regarding the optimal approach to endocrine therapy in both pre- and postmenopausal women are examined in this review.

Hormonal therapies have longplayed an important role inthe treatment of metastaticand early-stage breast cancer. Afterdemonstrating equivalent efficacy andless toxicity than high-dose estrogen,tamoxifen-a selective estrogen-receptormodulator (SERM)-has beenwidely used for the treatment of metastaticbreast cancer.[1] Multiple randomizedadjuvant trials subsequently demonstrated that patients treated withtamoxifen experienced fewer breastcancer recurrences, leading to its widespreaduse in the adjuvant setting.[2]

Neurotoxicity is a well-describedside-effect of paclitaxeltherapy, often characterizedas a peripheral sensory neuropathy.Neuropathy is a dose-dependenteffect, occurring with cumulative cyclesand higher doses. Occasionally,this may be dose-limiting for patientswho are benefiting from treatment, aswell as problematic for subsequenttherapies. Another well-recognizedthough less-described neurotoxic effectof paclitaxel is myopathy. Myopathy,consisting of myalgias andarthralgias, can be at least as commonwith standard paclitaxel regimens andequally troubling for patients. In thisissue of ONCOLOGY, Garrison andcolleagues review paclitaxel-associatedmyopathy and offer suggestionsfor patient management.

Drs. Fornier, Munster, and Seidman provide a comprehensive update of the management of metastatic breast cancer. They review the latest innovations in both chemotherapy and hormonal therapy for advanced disease, and demonstrate just how

Three oral 5-fluorouracil (5-FU) therapies have been approved by the US Food and Drug Administration or are in development for the treatment of patients with breast cancer: capecitabine, UFT, and 5-FU/eniluracil.

The treatment of refractory metastatic breast cancer is complex and challenging. Practicing oncologists must choose from an array of therapuetic options. Palliation remains the primary goal of treatment, and the risks and
Published: February 11th 2021 | Updated:

Published: April 30th 2025 | Updated:

Published: May 1st 2025 | Updated:

Published: May 2nd 2025 | Updated:

Published: May 1st 1999 | Updated:

Published: May 1st 2003 | Updated: