
Members of the committee reviewed findings from the phase 2/3 IMerge trial assessing imetelstat in patients with transfusion-dependent anemia in myelodysplastic syndromes.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Members of the committee reviewed findings from the phase 2/3 IMerge trial assessing imetelstat in patients with transfusion-dependent anemia in myelodysplastic syndromes.

Findings highlighted tumor reductions in patients with recurrent glioblastoma who received CART-EGFR-IL13Rα2 cells at 2 dose levels.

Findings from the phase 3 CheckMate 649 trial support nivolumab plus chemotherapy as a standard frontline therapy for patients with gastric, gastroesophageal junction, and esophageal adenocarcinoma.
Targeted imaging and biopsy may accurately identify patients with breast cancer who do not have residual disease, according to Henry Kuerer, MD, PhD, FACS, CMQ.

Treatment with brentuximab vedotin, lenalidomide, and rituximab yielded a progression-free survival benefit in the phase 3 ECHELON-3 trial.

Patient-reported outcomes support the clinical benefits of ide-cel among patients with relapsed/refractory multiple myeloma.

Treatment with simple hysterectomy reduces the incidence of urinary incontinence compared with radical hysterectomy in patients with low-risk cervical cancer.
Investigators plan to reinitiate enrollment of patients with non-Hodgkin lymphoma as part of the phase 1a/1b NX-2127-001 trial.

Reshma Jagsi, MD, DPhil, highlights disparities in hypofractionation, toxicity, and cardiac doses in radiotherapy for Black and Asian patients with breast cancer.

Findings from the INSITE trial support the Medical Imaging Drugs Advisory Committee’s positive opinion on pegulicianine for breast cancer surgery.

Findings from a phase 2 trial support the premise that activating the androgen receptor may elicit antitumor effects in patients with androgen receptor–positive, estrogen receptor–positive, HER2-negative breast cancer.

Developers also announced that they completed enrollment of patients with non–small cell lung cancer in the phase 2 THIO-101 trial.

Treatment with rusfertide may reduce the use of phlebotomy and limit debilitating disease-related symptoms in patients with polycythemia vera based on findings from the phase 2 REVIVE trial.

The DREAMM-8 trial assessing belantamab mafodotin plus pomalidomide/dexamethasone met the primary end point for those with relapsed/refractory multiple myeloma.

Findings from an open-label trial support the FDA approval of inotuzumab ozogamicin as a treatment for pediatric patients with relapsed/refractory acute lymphoblastic leukemia.
Investigators are evaluating IO-202 in patients with newly diagnosed chronic myelomonocytic leukemia as part of a phase 1 dose expansion trial.

Investigators are assessing avutometinib plus defactinib as a treatment for those with low-grade serous ovarian cancer as part of the phase 3 RAMP 301 trial.

Investigators will assess A2B530 as a treatment for patients with germline heterozygous HLA-A*02–positive colorectal cancer expressing carcinoembryonic antigen in the phase 1/2 EVEREST-1 trial.
Findings suggest that harnessing the tumoral node microenvironment may improve the ability to predict extracapsular nodal extensions in patients with oropharyngeal carcinoma.

Data from the phase 3 RUBY trial support the recommendation for dostarlimab plus chemotherapy for patients with advanced MSI-H/dMMR endometrial cancer.

Data from the TROPION-Lung01 and TROPION-Breast01 trials support the EU marketing authorization applications for datopotamab deruxtecan in non–small cell lung cancer and breast cancer, respectively.
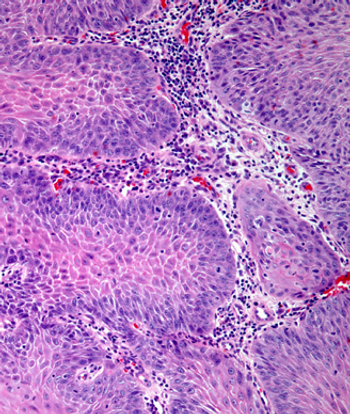
The role of multimodal approaches such as FDG-PET imaging may require further investigation in patients with human papillomavirus–positive oropharyngeal cancer, according to Samuel Regan, MD.

The regulatory agency has cleared developers to continue patient enrollment in the phase 2 IOV-LUN-202 trial evaluating LN-145 in non–small cell lung cancer.

Noa Biran, MD, speaks about recently approved bispecific therapies and current trials that may impact the standard of care in relapsed/refractory multiple myeloma.

Panitumumab plus modified FOLFOX6 appears to increase survival among patients with metastatic colorectal cancer and circulating tumor DNA that has no gene alterations in the PARADIGM trial.
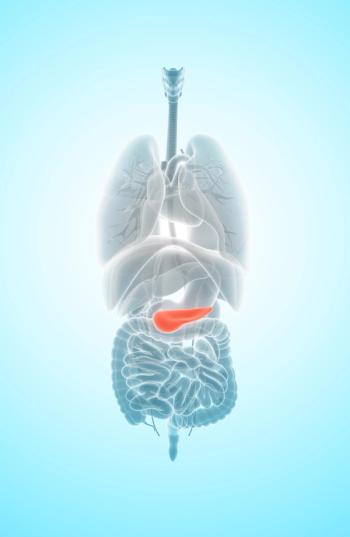
Higher CA19-9 levels appear to correlate with increased recurrence and mortality for specific patients with pancreatic cancer who undergo surgical resection.

Frontline pembrolizumab with or without chemotherapy appears to remain a standard of care for patients with recurrent or metastatic head and neck squamous cell carcinoma based on data from the LEAP-010 study.

Data from the phase 3 PAPILLON trial support the FDA approval of amivantamab plus chemotherapy for patients with metastatic non–small cell lung cancer harboring EGFR exon 20 insertion mutations.

Findings from the phase 3 DeFi trial support the marketing authorization application for nirogacestat as a treatment for adult patients with desmoid tumors in the European Union.

The nivolumab with chemotherapy regimen missed the primary end point on the phase 3 trial of patients with EGFR-mutated non–small cell lung cancer.