
Findings support cabozantinib plus atezolizumab as a potential new treatment option for those with metastatic castration-resistant prostate cancer that has progressed on a novel hormonal therapy.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Findings support cabozantinib plus atezolizumab as a potential new treatment option for those with metastatic castration-resistant prostate cancer that has progressed on a novel hormonal therapy.

Liver contrast-enhanced MRI resulted in a change in the local treatment plan for approximately a third of patients with colorectal liver metastases in the CAMINO study.

Treatment with pembrolizumab plus chemotherapy with or without bevacizumab yields an overall survival improvement regardless of squamous or nonsquamous cervical cancer histology.

Expanding or updating coverage with Medicaid may lead to positive health outcomes among patients with non–small cell lung cancer following surgery, according to Leticia Nogueira, PhD, MPH.

The FDA sets a Prescription Drug User Fee Action Date of November 16, 2024 for obe-cel as a treatment for those with relapsed/refractory B-cell acute lymphoblastic leukemia.

PTX-252 incorporates a novel molecular entity designed to enhance how cancer cells respond to chemotherapy.

The FDA grants clearance to an oral immobilization stent designed to redirect radiation to the target tumor area for patients with head and neck cancer.

Findings from the phase 1b/3 IMscin001 study support the European Commission’s approval of subcutaneous atezolizumab as a treatment for lung cancer and other disease types.

Findings from the phase 3 FRESCO-2 trial support fruquintinib’s potential to provide an improved survival benefit and quality of life for those with previously treated metastatic colorectal cancer.

Investigators are assessing avutometinib in combination with sotorasib as a treatment for those with KRAS G12C–mutated non–small cell lung cancer in the phase 1/2 RAMP-203 study.

Findings from a phase 1a/1b trial highlight that treatment with NX-5948 appears to be tolerable among patients with relapsed/refractory B-cell malignancies.
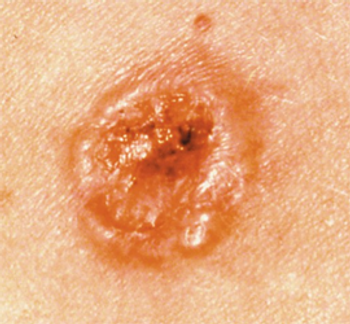
The DermaSensor device demonstrates a high rate of sensitivity in the detection of more than 200 types of skin cancers in a clinical study.
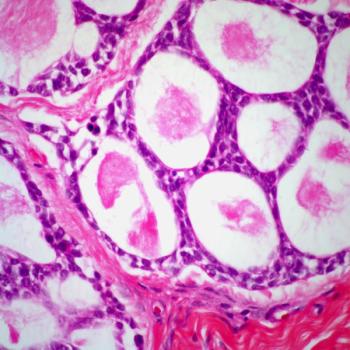
Certain patients with salivary gland carcinoma and close surgical margins may safely be considered for observation, according to findings from a retrospective cohort study.
Data from a case control study highlight a decrease in the risk of breast cancer among those with increasing sex hormone binding globulin concentrations.

Findings from a secondary analysis of a phase 3 trial support stereotactic radiosurgery as a standard of care for those with brain metastases, although whole-brain radiotherapy may yield more local and distant control.

Findings from a randomized trial highlight that treatment with mirtazapine may lead to an improvement in health-related quality of life among those with advanced non–small cell lung cancer and anorexia.

Adding adaptive radiation to chemotherapy may offer a novel approach to treating patients with advanced non–small cell lung cancer, according to Michael Steinberg, MD.

Treatment with imetelstat produces robust activity in patients with relapsed/refractory myelodysplastic syndrome regardless of ring sideroblasts in the phase 3 IMerge trial.

Adding pegylated L-asparaginase to consolidation chemotherapy demonstrates a modest decrease in minimal residual disease negativity among those with high-risk acute lymphoblastic leukemia.

Findings from the phase 2 JACKPOT8 study support golidocitinib as a potential treatment option for those with relapsed or refractory peripheral T-cell lymphoma.

Findings from the phase 3 KEYNOTE-A18 trial support the FDA approval of pembrolizumab plus external beam radiotherapy and concurrent chemotherapy in stage III to IVA cervical cancer.

The FDA has set a Prescription Drug User Fee Act date of June 29, 2024 for SH-105 as a treatment for patients with ovarian cancer or breast cancer.
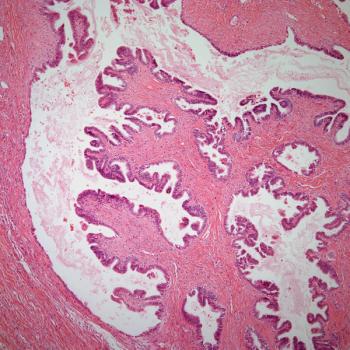
Investigators are assessing the safety and antitumor activity of rinatabart sesutecan in advanced epithelial ovarian cancer as part of the phase 1/2 PRO1184-001 study.

Findings from a retrospective study highlight an increase in the use of targeted agents like ibrutinib from 1998 to 2020 for American military veterans with chronic lymphocytic leukemia.

Investigators report high response rates with pirtobrutinib in those with mantle cell lymphoma and high-risk disease features in the phase 1/2 BRUIN trial.

The FDA cannot approve the application for zolbetuximab in advanced or metastatic gastric or gastroesophageal junction adenocarcinoma due to unresolved issues following a pre-license inspection of a third-party manufacturing site.

The FDA has set a Prescription Drug User Fee Act date of May 9, 2024 for the potential full approval of tisotumab vedotin for those with recurrent or metastatic cervical cancer.
Treatment with asciminib produces a favorable safety profile among patients with Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase.

Treatment with iopofosine I 131 appears to be well tolerated among patients with relapsed/refractory Waldenström macroglobulinemia.
Treatment with atezolizumab monotherapy yields an acceptable toxicity profile compared with chemotherapy among those with advanced or metastatic urothelial carcinoma.