
Data from a phase 2 trial support lenalidomide plus rituximab as a novel treatment strategy for patients with previously untreated mantle cell lymphoma.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Data from a phase 2 trial support lenalidomide plus rituximab as a novel treatment strategy for patients with previously untreated mantle cell lymphoma.
Adding all-trans retinoic acid to chemotherapy does not appear to improve complete remission rates compared with chemotherapy alone in patients with NPM1-mutated acute myeloid leukemia.
Survival following relapse appears to be shorter in patients with stage III colon cancer harboring KRAS exon 2 and BRAF V600E mutations regardless of microsatellite stability status.

Patients with extensive-stage small cell lung cancer who receive trilaciclib appear to experience lower rates of hematologic adverse effects than those who are not treated with the agent.
Treatment with dostarlimab plus chemotherapy followed by dostarlimab appears to improve outcomes among patients with advanced endometrial cancer and mismatch repair deficient disease in the phase 3 RUBY trial.

Data from a retrospective study spotlight a need for more effective first-line and second-line clinical management strategies to improve outcomes in patients with lower-risk myelodysplastic syndrome.

Data from a systematic review highlight a significantly higher risk of any bleeding and major bleeding in patients with treatment-naïve chronic lymphocytic leukemia treated with a second generation Bruton tyrosine kinase inhibitors.

More than half of resected patients with muscle-invasive urothelial carcinoma achieve a pathologic response with durvalumab plus neoadjuvant cisplatin/gemcitabine in the phase 2 SAKK 06/17 trial.

Investigators of the phase 3 KEYNOTE-671 trial identify no new safety signals with pembrolizumab plus chemotherapy in those with early-stage non–small cell lung cancer.

Collaboration among surgical specialties allows for safe removal of all visible tumors, thus improving survival in patients with advanced ovarian cancer, says Donal J. Brennan, MB, PhD.

The FDA has set a Prescription Drug User Fee Act date of April 30, 2024 for tovorafenib as a treatment for pediatric patients with relapsed or progressive low-grade glioma.
Mirvetuximab soravtansine-gynx may have the potential to become a new standard for patients with folate receptor α–positive, platinum-resistant ovarian cancer, experts say.

Patients with breast cancer harboring BRCA1/2 mutations appear to be more likely to undergo mastectomies than those without, according to Zachary Kiss, DO.

Data from the phase 3 MIRASOL trial support the European marketing authorization application for mirvetuximab soravtansine as a treatment for folate receptor α–positive, platinum-resistant ovarian cancer.
The regulatory agency has set a Prescription Drug User Fee Act date of April 23, 2024 for N-803 plus BCG as a treatment for BCG-unresponsive, non–muscle invasive bladder carcinoma.
Data from the phase 3 RATIONALE-301 trial appear to support tislelizumab as a potential frontline treatment option for patients with unresectable hepatocellular carcinoma.

Data from the phase 3 JUPITER-02 study and phase 2 POLARIS-02 study support the FDA approval of toripalimab-tpzi alone and in combination with gemcitabine and cisplatin in nasopharyngeal carcinoma.
Paige Lymph Node is the first artificial intelligence application of its kind to receive breakthrough device designation from the FDA for detecting breast cancer metastases.
Investigators will assess the safety and preliminary anti-tumor activity of ONCT-534 in metastatic castration-resistant prostate cancer in the phase 1/2 study ONCT-534-101.

Findings from a phase 2 trial also show that there is no progression-free survival or overall survival benefit with lapatinib plus chemoradiotherapy in patients with non-human papillomavirus–related head and neck cancer.

The primary end point of overall response rate is met in the phase 2 ROSEWOOD study of patients with relapsed/refractory follicular lymphoma receiving zanubrutinib plus obinutuzumab vs obinutuzumab monotherapy.

Data from a phase 2 trial suggests that earlier use of abiraterone acetate/prednisone plus cabazitaxel may elicit clinical benefit in patients with metastatic castration-resistant prostate cancer.

A yoga intervention program in patients with head and neck cancer appears to correlate with significantly fewer feeding tube placements compared with those receiving usual care.

ERBB2, FAT3, and FRS2 alterations appear to correlate with improved progression-free survival with ribociclib compared with placebo in patients with advanced breast cancer.

Treatment with mRNA-4157 plus pembrolizumab yields benefits in resected melanoma subgroups in the phase 2 KEYNOTE-942 trial, including those with BRAF-mutated tumors.

Central nervous system progression-free survival appears to be longer with trastuzumab deruxtecan than with comparator treatment in those with HER2-positive metastatic breast cancer and brain metastases.
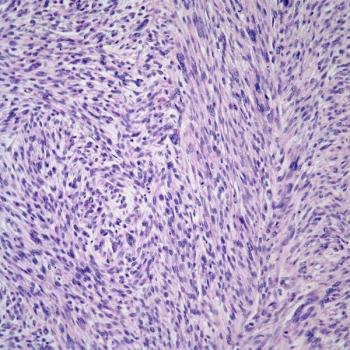
Findings from a secondary data analysis suggest a need for further research to determine risk factors that place younger patients who are Puerto Rican at a high risk of endometrial cancer.

Adding durvalumab to standard-of-care chemotherapy appears to improve downstaging in patients with resectable gastric cancer and gastroesophageal junction cancer in the phase 3 MATTERHORN study.

Data from a phase 1a/1b trial support additional research on dual TIGIT/PD-L1 inhibition in patients with advanced solid tumors.
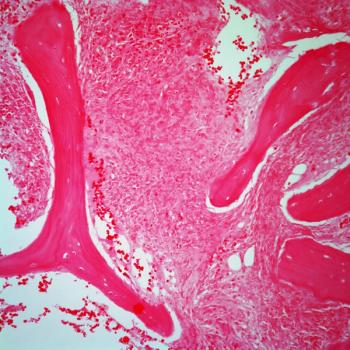
Data from the phase 3 ECHELON-1 trial support the European Commission’s approval of brentuximab vedotin plus chemotherapy as a treatment for those with CD30-positive stage III Hodgkin lymphoma.