
Characteristics including Ogata score and phenotypic aberrancies may correlate with mutational patterns that are predictive of treatment response in patients with myelodysplastic syndrome.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Characteristics including Ogata score and phenotypic aberrancies may correlate with mutational patterns that are predictive of treatment response in patients with myelodysplastic syndrome.

Findings from the phase 3 THOR study support the marketing authorization application for erdafitinib as a treatment for those with advanced or metastatic urothelial cancer harboring FGFR3 alterations.

Mohammad Bakri Hammami, MD, highlights a need to address socio-racial disparities among Black and non-Black patients with primary myelofibrosis to ensure that everyone receives high-quality treatment.

Data from the phase 3 ASCEND trial and a phase 1/2 trial support the National Medical Products Administration’s approval of acalabrutinib in China as a treatment for chronic lymphocytic leukemia.

Patients with dyserythropoiesis appear to have higher PD-L1 expression than those without in a study population of those with myelodysplastic syndromes.

Real-world data suggest that the presence of pelvic lymphadenopathy should not be viewed as a contraindication to trimodality therapy in nonmetastatic clinically node-positive bladder cancer.
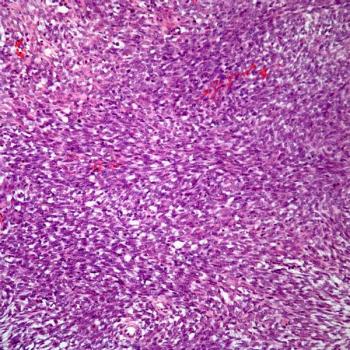
The safety profile of tisotumab vedotin-tftv among those with recurrent or metastatic cervical cancer in the phase 3 innovaTV 301 trial appears to be comparable with prior reports of the agent.

Results from a randomized trial suggest that neoadjuvant FOLFOX may be an effective treatment for patients with locally advanced rectal cancer who are suitable to undergo sphincter-sparing surgery.

Findings from a real-world cohort study may support renewed attention to completing molecular genotyping before first-line therapy for patients with metastatic nonsquamous non–small cell lung cancer.

Investigators report no new safety signals with alectinib as a treatment for those with early-stage, ALK-positive non–small cell lung cancer in the phase 3 ALINDA study.

Jonathan Leeman, MD, indicates that the longer-follow is necessary to confirm late toxicities and disease control associated with adaptive radiotherapy in patients with prostate cancer.

Combining tucatinib with ado-trastuzumab emtansine does not appear to result in any new safety signals in the treatment of those with locally advanced or metastatic HER2-positive breast cancer.

Investigators report that patients with relapsed/refractory myeloma treated with mezigdomide and dexamethasone primarily experienced myelotoxic effects.
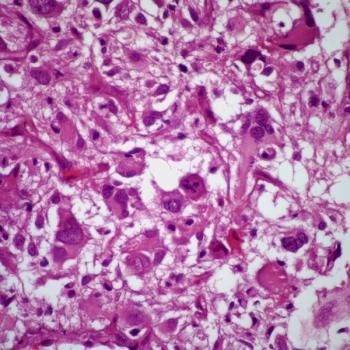
Investigators also report an improvement in time to next intervention with vorasidenib compared with placebo among patients with IDH-mutated glioma in the phase 3 INDIGO trial.

Findings from a prospective study indicate that patients with undifferentiated pleomorphic sarcoma and leiomyosarcoma may present similarly to elderly patients with osteosarcoma.

Findings from a multi-center phase 1/2 trial highlight only that only grade 1 cytokine release syndrome events occurred among patients with indolent non-Hodgkin lymphoma receiving MB-106.

Treatment with divarasib appears to produce a manageable safety profile of low-grade toxicities in patients with advanced or metastatic KRAS G12C–mutated solid tumors.
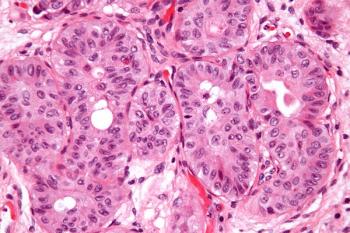
Data from the phase 3 THOR study support the supplemental biologics license application for erdafitinib in previously treated advanced or metastatic urothelial carcinoma with FGFR3 alterations.

Findings from an exploratory subgroup analysis in the phase 3 INNOVATE-3 trial suggest that Tumor Treating Fields plus paclitaxel may confer a survival benefit in patients who received only 1 prior line of therapy for platinum-resistant ovarian cancer.

Updated findings from the phase 3 TROPiCS-02 trial support sacituzumab govitecan as a standard treatment for hormone receptor–positive, HER2-negative breast cancer, according to Sara Tolaney, MD, MPH.

The safety profile of cabozantinib as a treatment for patients with advanced pancreatic neuroendocrine tumors in the phase 3 CABINET trial is comparable with previous reports of the agent.

Findings from the phase 1/2 MajesTEC-1 study support the European Commission’s approval of teclistamab at a reduced dose frequency for patients with relapsed/refractory multiple myeloma.
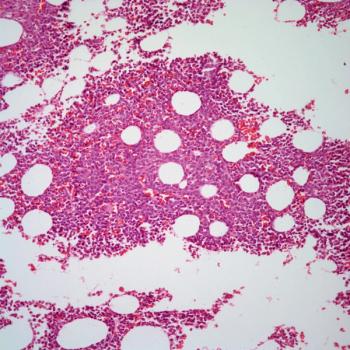
Patients who are already enrolled on clinical trials assessing magrolimab in acute myeloid leukemia are eligible to continue treatment with the agent.

Data from the phase 3 PAPILLON trial support the supplemental biologics license application for amivantamab plus chemotherapy as a treatment for EGFR exon 20 insertion mutation–positive non–small cell lung cancer.

Investigators will assess the safety and pharmacodynamics of ALE.C04 in patients with CLDN1-positive head and neck squamous cell carcinoma as part of a phase 1/2 clinical trial.

Findings from a real-world study highlight that treatment utilization, such as agents for managing tumor lysis syndrome, appears to be more intense when older patients with chronic lymphocytic leukemia initiate treatment with venetoclax plus obinutuzumab.
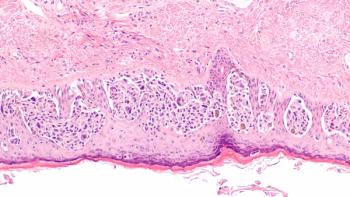
Data from the phase 3 CheckMate-76k trial support the European Commission’s approval of nivolumab as an adjuvant treatment for patients with resected stage IIB or IIC melanoma.
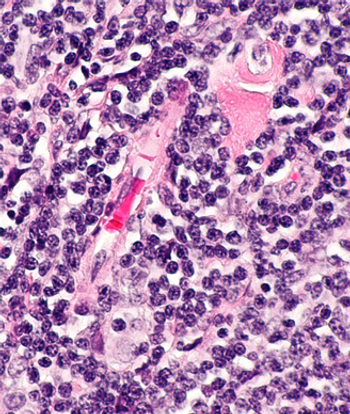
Data from the phase 1 ELM-1 trial and phase 2 ELM-2 trial support the marketing authorization application for odronextamab in relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma.
The FDA sets a Prescription Drug User Fee Act date in the fourth quarter of 2023 for its decision regarding enzalutamide as a treatment for those with non-metastatic hormone-sensitive prostate cancer with a high-risk of biochemical recurrence.

Findings from the phase 2/3 IMerge trial support the new drug application for imetelstat in the treatment of patients with transfusion-dependent anemia in lower-risk myelodysplastic syndrome.