
Longitudinal ctDNA assessments may be a relevant tool for making treatment decisions for patients with EGFR-mutated non–small cell lung cancer based on findings from the phase 2 APPLE trial.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Longitudinal ctDNA assessments may be a relevant tool for making treatment decisions for patients with EGFR-mutated non–small cell lung cancer based on findings from the phase 2 APPLE trial.

The regulatory agency has set a Prescription Drug User Fee Act date of February 7, 2024 for pembrolizumab plus chemotherapy as a treatment for patients with advanced or metastatic biliary tract cancer.

Findings from the phase 3 CARTITUDE-4 study support the supplemental biologics license application for ciltacabtagene autoleucel in the treatment of relapsed/refractory multiple myeloma.

Treatment with eftilagimod alpha in combination with pembrolizumab appears safe and well tolerated among patients with metastatic head and neck squamous cell carcinoma in the phase 2 TACTI-002 study.

Maintenance bevacizumab plus durvalumab and olaparib appears to produce a progression-free survival benefit among those with HRD-negative advanced ovarian cancer in the phase 3 DUO-O trial.
Tucatinib plus trastuzumab represents a valuable, novel option for HER2-positive, RAS wild-type, unresectable or metastatic colorectal cancer that is chemotherapy refractory, according to the authors of the phase 2 MOUNTAINEER trial.
The Molecular International Prognostic Scoring System appears to improve prognostic discrimination across all clinical end points compared with other scores in patients with myelodysplastic syndromes.

Zanubrutinib appears to produce significantly better progression-free survival and overall cardiac safety compared with ibrutinib in the treatment of patients with relapsed/refractory chronic lymphocytic leukemia.

The safety review committee of the phase 1/2 Acclaim-1 trial identifies a recommended phase 2 dose of quaratusugene ozeplasmid plus osimertinib in the management of advanced non–small cell lung cancer.

Data from the phase 3 CheckMate 816 trial support the European Medicines Agency’s Committee for Medicinal Products for Human Use recommendation to approve nivolumab plus platinum-based chemotherapy as a treatment for resectable non–small cell lung cancer.

Fifteen-year follow-up data suggest the importance of considering the trade-offs between risks and benefits of active monitoring, prostatectomy, and radiotherapy for localized prostate cancer.

The FDA sets a Prescription Drug User Fee Act date of November 25, 2023 for lifileucel in the treatment of patients with advanced melanoma.
Findings from the registrational phase 1/2 TRIDENT-1 trial support the new drug application for repotrectinib in the treatment of those with ROS1-positive advanced or metastatic non–small cell lung cancer.

Data from the phase 3 DUO-E trial indicate that the safety of durvalumab/chemotherapy with or without olaparib/durvalumab or durvalumab monotherapy maintenance in recurrent endometrial cancer was consistent with previous reports of each agent.

Findings from the phase 3 DAWNA-2 trial suggest that dalpiciclib plus endocrine therapy may be a potential novel first-line treatment option for hormone receptor–positive, HER2-negative advanced breast cancer.
Data from the phase 3 FRESCO and FRESCO-2 trials support the new drug application for fruquintinib as a treatment for patients with previously treated metastatic colorectal cancer.

Patients with nonsquamous non–small cell lung cancer experiencing clinical benefit with sitravatinib plus nivolumab in the phase 3 SAPPHIRE trial are eligible to remain on treatment.

Findings from a study suggest that anti-frameshift peptide antibodies may also predict incidence of immune-related adverse effects in patients with lung cancer following immune checkpoint inhibitor therapy.

Supporting data for the supplemental new drug application for toripalimab plus chemotherapy in recurrent metastatic triple-negative breast cancer come from the phase 3 TORCHLIGHT study.

Investigators plan to initiate a phase 2 study evaluating AFM13 plus AB-101 in patients with relapsed/refractory classical Hodgkin lymphoma in the first half of 2024.
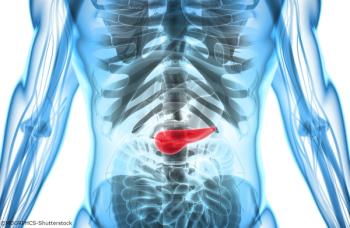
Mitazalimab is currently under investigation in combination with chemotherapy as a treatment for patients with metastatic pancreatic ductal adenocarcinoma in the phase 2 OPTIMIZE-1 trial.

Asciminib does not appear to interfere with the general life activities of patients with resistant/intolerant chronic phase chronic myeloid leukemia in the phase 3 ASCEMBL trial.

Hospitalization rates appear to be comparable between patients receiving trastuzumab deruxtecan and trastuzumab emtansine for HER2-positive breast cancer in the phase 3 DESTINY-Breast03 study.
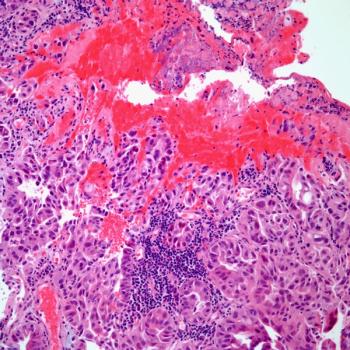
Investigators say that they do not have any safety concerns regarding GEN-001 and avelumab in patients with advanced gastric or gastroesophageal junction adenocarcinoma.

Adult patients with indolent systemic mastocytosis can now receive avapritinib following the FDA’s approval of the agent.
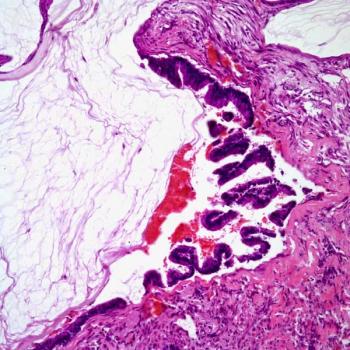
Findings from a retrospective analysis highlight no significant differences in metastasis-free survival in patients with muscle-invasive bladder cancer who received radical cystectomy vs trimodality therapy.
Santosh Rao, MD, discusses ongoing and potential future research efforts aiming to maximize the benefits that patients with kidney cancer may derive from integrative oncology treatment techniques.

Santosh Rao, MD, discusses guideline recommendations and therapeutic techniques that underscore the current integrative oncology landscape for patients with kidney cancer.

Patients with relapsed/refractory diffuse large B-cell lymphoma can now receive epcoritamab following the FDA’s approval of the agent.
The addition of cabozantinib to nivolumab and ipilimumab also appears to improve responses in patients with advanced renal cell carcinoma in the phase 3 COSMIC-313 trial.