Incorporating PSMA PET into pre-surgical risk assessment may help urologists determine whether surgery should be performed on patients with advanced prostate cancer, according to Loïc Djaïleb, MD, PhD.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Incorporating PSMA PET into pre-surgical risk assessment may help urologists determine whether surgery should be performed on patients with advanced prostate cancer, according to Loïc Djaïleb, MD, PhD.

Findings from the phase 3 RUBY trial support the FDA’s approval of dostarlimab/chemotherapy as a treatment for advanced or recurrent endometrial cancer that is either mismatch repair deficient or microsatellite instability–high.

The regulatory agency requests enhanced product testing and additional controls in their market application review of denileukin diftitox for the treatment of those with relapsed/refractory cutaneous T-cell lymphoma.
Investigators of a phase 1 study evaluating cosibelimab in patients with locally advanced or metastatic cutaneous squamous cell carcinoma plan to share updated results at a future medical conference.

Treatment with neoadjuvant pembrolizumab among those with estrogen receptor–positive, HER2-negative breast cancer in the phase 3 KEYNOTE-756 trial does not produce any new safety signals.
Brian Van Tine, MD, PhD, discusses clinical trials assessing several regimens that may lead to novel treatment options for those with desmoid tumors, dedifferentiated liposarcoma, and other hard-to-treat sarcoma subtypes.

The safety profile of trastuzumab deruxtecan among patients with HER2-expressing solid tumors in the phase 2 DESTINY-PanTumor02 trial appears to be comparable with previous reports of the agent.

Investigators will submit data from the phase 3 ENHANCE trial evaluating magrolimab plus azacitidine in higher-risk myelodysplastic syndromes for presentation at a future medical meeting.

Findings from the phase 3 KEYNOTE-811 trial support the CHMP’s recommendation to approve pembrolizumab plus trastuzumab in HER2-positive advanced gastric or gastroesophageal junction adenocarcinoma.

Data from the phase 3 QuANTUM-First trial support the FDA’s approval of quizartinib for managing FLT3-ITD–positive acute myeloid leukemia.
The safety profile of pembrolizumab among patients with high-risk locally advanced cervical cancer in the phase 3 KEYNOTE-A18 trial appears to be consistent with previous reports of the agent.

The investigational antibody-drug conjugate ARX517 is currently under evaluation in patients with metastatic castration-resistant prostate cancer as part of the phase 1/2 APEX-01 trial.

Findings from the REBECCA trial support additional research for nurse navigation intervention for managing psychological vulnerability among patients with breast cancer.

Selinexor plus ruxolitinib is under investigation as a treatment for JAK inhibitor-naïve patients with myelofibrosis as part of the phase 3 XPORT-MF-034 trial.
The new drug application for rivoceranib plus camrelizumab in unresectable hepatocellular carcinoma is supported by findings from the phase 3 CARES 310 study.

The QPOLE assay may be a fast, low-cost alternative to other next-generation sequencing tools for POLE testing among patients with endometrial cancer.

Findings from the phase 3 KEYNOTE-564 trial support adjuvant pembrolizumab following nephrectomy as a standard-of-care treatment for patients with clear cell renal cell carcinoma.
The improvements occur regardless of PD-L1 status among patients receiving lenvatinib plus pembrolizumab for advanced renal cell carcinoma in the phase 3 CLEAR study.
Using a laser ablation system, imaging mass cytometry is designed to measure over 40 metal isotopes in a tumor sample to identify how immune cells in clear cell renal cell carcinoma are organized.
Factors including measurable residual disease and CAR T-cell expansion appear to predict duration of response following JCAR014 in those with relapsed or refractory chronic lymphocytic leukemia in a phase 1/2 trial.

Findings from the phase 1b KRYSTAL-1 trial support the continued development of adagrasib in the treatment of patients with KRAS G12C–mutated non–small cell lung cancer and central nervous system metastases.

Epcoritamab is a treatment option for patients with relapsed/refractory diffuse large B-cell lymphoma that was approved by the FDA based on findings from the phase 1/2 EPCORE NHL-1 trial.

Data from the phase 2 ROCKstar trial support the approval of belumosudil as a treatment for patients with chronic graft-versus-host-disease in Scotland.

Combining enfortumab vedotin with pembrolizumab yields a manageable safety profile in patients with locally advanced or metastatic urothelial cancer in the phase 1b/2 EV-103 study.

Findings from the phase 3 FRESCO-2 trial support fruquintinib plus best supportive care as a global treatment option for patients with refractory metastatic colorectal cancer.

Patients with select hematologic cancers, including lymphoma and multiple myeloma, appear to benefit from a ready-to-dilute formation of intravenous cyclophosphamide, the new drug application for which was approved by the FDA.

Data from the phase 2 MANIFEST study suggest that pelabresib plus ruxolitinib may improve the standard of care for patients with myelofibrosis who are naïve to treatment with JAK inhibitors.
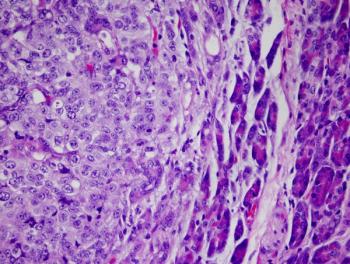
The benefit of nivolumab/ipilimumab plus single-agent nivolumab for unresectable advanced melanoma with poor prognostic characteristics was comparable in all-comers and patients with brain metastases.

Hypersensitivity reactions following platinum-based treatment appear to be more severe than hypersensitivity reactions after taxanes among patients with cancer.
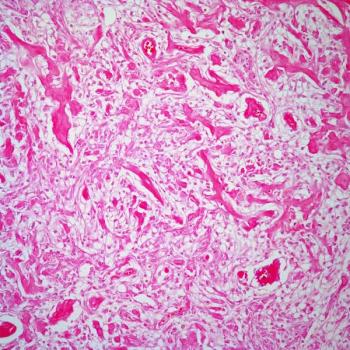
Overall survival is expected to continue improving among patients with glioblastoma being treated with frontline olaptesed pegol plus radiotherapy and bevacizumab who remain on the phase 1/2 GLORA study.