
Safety findings from the phase 3 FLAURA2 trial appear to be consistent with the known profiles of osimertinib and chemotherapy for the treatment of those with EGFR-mutated non–small cell lung cancer.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Safety findings from the phase 3 FLAURA2 trial appear to be consistent with the known profiles of osimertinib and chemotherapy for the treatment of those with EGFR-mutated non–small cell lung cancer.

Investigators are expected to share initial safety and efficacy data from a phase 1/2 trial assessing IMPT-314 as a treatment for aggressive B-cell lymphoma in the second half of 2023.

Prexasertib is currently under investigation as part of a phase 2 trial as a treatment for patients with platinum-resistant ovarian cancer, endometrial adenocarcinoma, and urothelial cancers.

The FDA requests additional information requiring time and resources extending beyond the current evaluation period for vic-trastuzumab duocarmazine in advanced HER2-positive breast cancer.
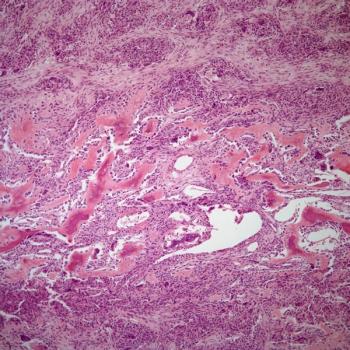
Findings from a phase 2 trial suggest that trabectedin plus radiotherapy is a tolerable treatment option in patients with myxoid liposarcoma despite yielding an unsatisfactory overall response rate.
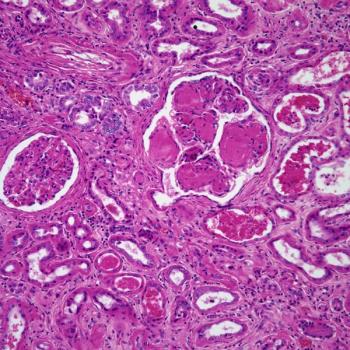
Findings from the phase 1/2 MajesTEC-1 trial suggest that preemptively planning and promptly managing cytokine release syndrome with supportive care in patients with multiple myeloma treated with T-cell–engaging bispecific antibodies may be beneficial.

Although findings from a population-based meta-analysis suggest a greater risk of death in patients due to cancers diagnosed during pregnancy and postpartum, not all disease sites had the same risk.

The FDA requests additional data and a safety update regarding N-803 plus BCG as a treatment for patients with BCG-unresponsive non-muscle invasive bladder carcinoma.

Findings from a nested case-control study may enable individualized colorectal cancer risk estimations for Hodgkin lymphoma survivors who receive radiotherapy plus procarbazine.
China’s National Medical Products Administration has also approved zanubrutinib as a treatment for patients with Waldenström macroglobulinemia based on findings from the phase 3 ASPEN trial.

Findings from the phase 3 ORIENT-31 trial support the use of sintilimab plus chemotherapy as a potential novel treatment strategy for those with EGFR-mutated nonsquamous non–small cell lung cancer.

Investigators will assess INB-400 as a treatment for patients with newly diagnosed glioblastoma in a phase 2 trial expected to initiate in the second half of 2023.
Treatment with isolated hepatic perfusion appears to yield improved progression-free survival compared with best alternative care in patients with isolated uveal melanoma liver metastases.

An ancillary analysis of 2 clinical trials finds that pathologic complete response may be a prognostic factor of clinical outcomes in soft tissue sarcoma in future studies.

An artificial intelligence-based model for detecting lymph node metastases appears to identify tumor micrometastases in bladder cancer that pathologists may miss while classifying patient results as negative.

Combination treatment with IMX-110 and tislelizumab yields no dose-limiting toxicities thus far in the first cohort of patients with advanced/metastatic colorectal cancer in the phase 1b/2a IMMINENT-01 trial.

The FDA’s approval of the FoundationOne Liquid CDx assay may improve access to treatment with mobocertinib for patients with non–small cell lung cancer harboring EGFR exon 20 insertion mutations.

Based on findings from the phase 3 MIRASOL trial, investigators plan to submit a supplemental biologics license application for mirvetuximab soravtansine in folate receptor α–positive platinum-resistant ovarian cancer.

According to the investigators of the INSITE trial, the potential benefit of pegulicianine fluorescence–guided surgery in breast cancer warrants investigation in future studies.
ERAS-801, which now has FDA fast track designation for glioblastoma with EGFR alterations, is currently under investigation as a monotherapy in the phase 1 THUNDERBBOLT-1 trial.
Patients in Canada who have advanced non–small cell lung cancer can now receive cemiplimab plus chemotherapy as a first-line treatment following Health Canada’s approval of the regimen.

THIO plus cemiplimab does not appear to yield any dose-limiting toxicities or significant treatment-related adverse effects in patients with advanced non–small cell lung cancer.

Enzalutamide with or without leuprolide also reduces the risk of prostate-specific antigen progression in those with non-metastatic hormone-sensitive prostate cancer in the phase 3 EMBARK trial.

Liso-cel produces no new safety signals in relapsed or refractory mantle cell lymphoma and follicular lymphoma in the phase 1 TRANSCEND NHL 001 trial and the phase 2 TRANSCEND FL trial.

Data from the phase 3 COMMANDS trial support a supplemental biologics license application for an expanded indication for luspatercept-aamt for lower-risk myelodysplastic syndrome with anemia.
Data from the phase 3 NRG-RTOG 0631 may inform future research assessing spinal radiosurgery in the oligometastatic setting, according to an expert from Stony Brook University Medical Center.

The addition of short-term androgen deprivation to dose-escalated radiation therapy did not yield a significant difference in quality-of-life outcomes vs radiotherapy alone for those with intermediate-risk prostate cancer, according to an expert from Henry Ford Health Cancer.
Data from the phase 2 TITAN-TCC trial suggest that early non-responders with metastatic urothelial carcinoma with PD-L1–positive tumors benefit from nivolumab plus nivolumab/ipilimumab boosts.
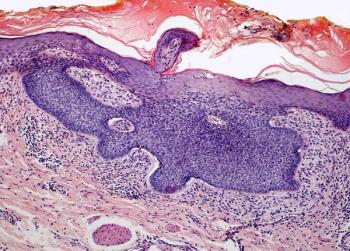
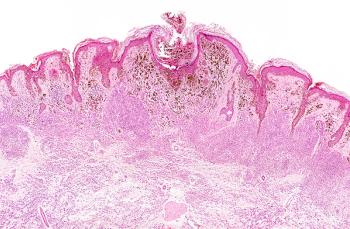
The 31-gene expression profile test appears to allow for significant and independent risk stratification of those with stage I cutaneous melanoma.
Results from a phase 2 study support additional clinical trials assessing belzutifan-based regimens for patients with advanced clear cell renal cell carcinoma.