
Findings from the phase 3 TRANSFORM study support the Committee for Medicinal Products for Human Use’s recommendation to approve lisocabtagene maraleucel for large B-cell lymphoma.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Findings from the phase 3 TRANSFORM study support the Committee for Medicinal Products for Human Use’s recommendation to approve lisocabtagene maraleucel for large B-cell lymphoma.

Supporting data for the biologics license application for trastuzumab biosimilar HLX02 in HER2-overexpressing cancers come from comparative analytical studies and a global phase 3 trial.
Progression-free survival and overall survival appear to be worse in patients with metastatic colorectal cancer who have a high- vs low-BRAF allele fraction after treatment with a BRAF inhibitor plus an anti-EGFR agent plus or minus a MEK inhibitor.

The FDA’s decision to lift a partial clinical hold on the phase 1/2 VELA trial follows reports of visual adverse effects in patients with advanced solid tumors in February 2023.
Retrospective data encourage further exploration of non-ASCT regimens in younger patients with mantle cell lymphoma, while maintenance rituximab should be considered following first-line therapy with bendamustine plus rituximab.

The FDA has set a Prescription Drug User Fee Act date in the fourth quarter of 2023 for encorafenib plus binimetinib as a treatment for BRAF V600E–mutant metastatic non–small cell lung cancer.
Acceptance of an investigational new drug application for GRC 54276 will allow investigators to proceed with a phase 1/2 trial evaluating the agent in patients with advanced solid tumors and lymphomas.
Data from a Chinese phase 1/2 trial suggest that osemitamab monotherapy may yield prolonged responses in Claudin18.2-expressing pancreatic cancer.

Patients with non–small cell lung cancer who had mild/moderate immune-related adverse effects appear to have improved overall survival compared with those without following atezolizumab-based therapy.

The 10-year cumulative local recurrence rate in patients with early breast cancer receiving accelerated partial breast irradiation with multi-catheter brachytherapy does not significantly differ from those receiving whole-breast irradiation following breast-conserving surgery.

ISB 1442, which received orphan drug designation from the FDA, is currently under investigation in a first-in-human phase 1/2 trial as a treatment for patients with relapsed/refractory multiple myeloma.
Investigators are assessing tirabrutinib, which received orphan drug designation from the FDA, as a treatment for patients with relapsed or refractory primary central nervous system lymphoma in the phase 2 PROSPECT study.
Overall survival after 10 years appears to be comparable regardless of whether patients 65 years or older received irradiation following surgery for low-risk, hormone receptor–positive breast cancer.

Findings from 2 clinical trials support the approval of acalabrutinib as a treatment for patients with mantle cell lymphoma in China.
Treatment with retroperitoneal lymph node dissection for testicular seminoma with limited retroperitoneal lymphadenopathy is associated with low long-term morbidity.

Adding avelumab to carboplatin plus paclitaxel appears to elicit a progression-free survival advantage compared with chemotherapy alone in those with advanced or recurrent endometrial cancer.

Efficacy and safety findings from the phase 3 GLOW trial assessing zolbetuximab and CAPOX in CLDN18.2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma were consistent with the phase 3 SPOTLIGHT trial, according to an expert from Weill Cornell Medical College

Findings from a cohort of the Childhood Cancer Survivor Study identify a prediction model that may accurately identify childhood cancer survivors at varying risks of late kidney failure.
Human papillomavirus and p16 discordance may correlate with a worse prognosis for oropharyngeal cancer, according to data from an individual patient data analysis.

A genetic analysis indicates that multi-ancestry polygenic risk scores may be “potentially useful” in detecting risk of aggressive prostate cancer in patients of African ancestry, according to an expert from the University of Southern California.

According to phase 1b/2 HPV001 study data, VTP-200 appears to produce no serious adverse effects in patients with low-grade cervical human papillomavirus lesions.

Investigators plan to submit a communication application to seek marketing approval in China for ARX788 as therapy for HER2-positive advanced or metastatic breast cancer.

Investigators are assessing FORE8394, which received orphan drug designation from the FDA, in primary brain and central nervous system malignancies in the phase 2 FORTE trial.
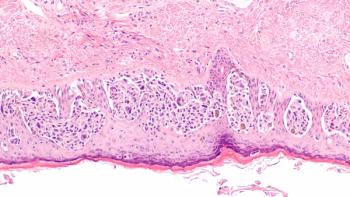
Combination treatment with nivolumab and relatlimab appears to be a safe treatment option for patients with advanced melanoma who have progressed on prior anti–PD-L1 therapy.

Data from the phase 2 INNATE trial indicate that pimivalimab plus JTX-8064 also appears to be well tolerated in patients with platinum-resistant ovarian cancer.
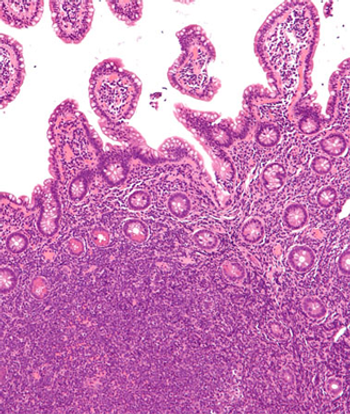
Investigators indicate that although avoiding efficient mantle cell lymphoma treatment due to late effects may seem okay, most long-term health care needs are disease related in patients up to 70 years.

Use of a kit that prepares for gallium Ga 68 gozetotide injection in the phase 3 VISION study affirms its ability to identify patients who are suitable to receive PSMA-based radioligand for metastatic prostate cancer.

Findings from a confirmatory factor analysis appear to validate the structure of the Quality of Life in Myelodysplasia Scale in myelodysplastic syndromes.

Factors including age, gender, and facility type may influence the likelihood of pain specialist referrals for lung cancer, suggesting a need for multidisciplinary pain management in this patient population.
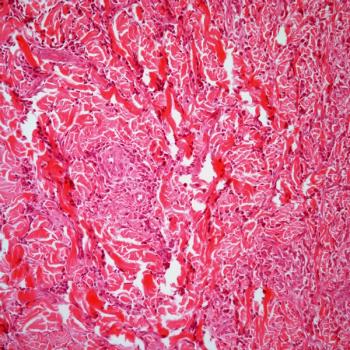
EP0042 shows promising early clinical efficacy in patients with pretreated acute myeloid leukemia, according to investigators.