
The FDA approved adjuvant pembrolizumab for the treatment of patients with resected non–small cell lung cancer, based on data from the phase 3 KEYNOTE-091/EORTC-1416-LCG/ETOP-8-15-PEARLS trial.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

The FDA approved adjuvant pembrolizumab for the treatment of patients with resected non–small cell lung cancer, based on data from the phase 3 KEYNOTE-091/EORTC-1416-LCG/ETOP-8-15-PEARLS trial.

Black and Hispanic patients are less likely to receive opioids than their White counterparts; bias training and logistical support may act as potential strategies to mitigate these disparities, according to an expert from Dana-Farber Cancer Institute.

Treatment with avutometinib and defactinib in patients with low-grade serous ovarian cancer resulted in a positive objective response rate.

Treatment with nivolumab plus ipilimumab appears to result in overall survival and progression-free survival benefit in patients with intermediate- or poor-risk sarcomatoid renal cell carcinoma.

DB-1303, an investigational third generation antibody-drug conjugate that now has FDA fast track designation, may benefit patients with HER2-overexpressing endometrial cancer.
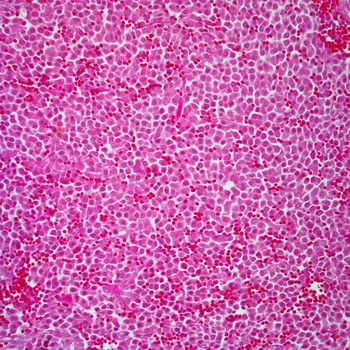
Data from the phase 2 PIONEER trial supports the supplemental new drug application for avapritinib in adult patients with indolent systemic mastocytosis, an uncontrolled proliferation and activation of mast cells.

Pre-operative tremelimumab plus durvalumab elicited encouraging safety and activity in patients with microsatellite instability–high resectable gastric or gastroesophageal junction cancer.

The FDA based its accelerated approval of tucatinib and trastuzumab combination therapy for HER2-positive metastatic colorectal cancer on results from the phase 2 MOUNTAINEER trial.
The FDA has given fast track designation to peptide-based immunotherapy agent EVX-01 in combination with pembrolizumab for metastatic melanoma.
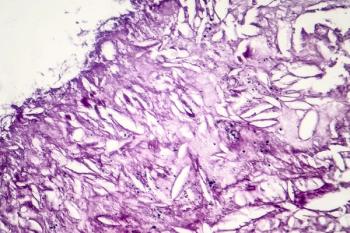
Cisplatin plus veliparib appears to improve progression-free survival among patients with BRCA-like metastatic triple-negative breast cancer, but not in those with non–BRCA-like metastatic breast cancer.

An expert from the University of Texas MD Anderson Cancer Center discussed kidney cancer treatment updates from a recent medical conference.

Findings from the phase 3 Neotorch trial indicate that toripalimab plus chemotherapy has met the primary end point of event-free survival among patients with operable non–small cell lung cancer.

Patients who are set to undergo autologous hematopoietic stem cell transplant following induction chemotherapy may have poor mobility across several domains vs an age-matched control group.

Findings from the phase 1/2 CA001-030 study indicate that BMS-986012 in combination with nivolumab is well tolerated among patients with small cell lung cancer.

The FDA grants orphan drug designation to investigational G-quadruplex transcription inhibitor QN-302 for pancreatic cancer.
An expert from Natera indicates that post-operative ctDNA level may have strong prognostic and predictive value in patients with resectable colorectal cancer.

LP-284, which now has orphan drug designation from the FDA for mantle cell lymphoma, yielded positive preclinical data at the 2022 American Society of Hematology Annual Meeting and Exposition.
Patients with HER2-positive breast cancer and triple-negative breast cancer may not require breast surgery if a pathological complete response can be identified using image-guided vacuum core biopsy following neoadjuvant systemic therapy.

During the 2022 Society for Urologic Oncology (SUO) Annual Meeting, an expert from the Baylor College of Medicine, discussed how perceptions around the use of testosterone therapy have evolved for patients with metastatic prostate cancer.

In a population of patients with patients with mantle cell lymphoma and follicular lymphoma, zandelisib plus zanubrutinib did not increase the rate or severity of class-related adverse effects.

Data from a meta-analysis suggest that single-fraction stereotactic ablative body radiotherapy could result in a lower incidence of local failure than multifractionated radiation in patients with renal cell carcinoma.
Lenalidomide plus rituximab consolidation therapy prior to bendamustine/rituximab induction did not significantly improve progression-free survival in patients with mantle cell lymphoma in the phase 2 NCTN E1411 trial.

The National Medical Products Administration of China gives approval to mobocertinib for patients with non–small cell lung cancer harboring EGFR exon 20 insertion mutations.

Patients with metastatic prostate cancer and dysphagia or difficulty swallowing may benefit from TAVT-45 compared with abiraterone acetate tablets.
Findings from the phase 1b/2 NEXICART-1 study indicated that NXC-201 produced complete responses in 6 evaluable patients with relapsed or refractory AL amyloidosis.
Findings from the phase 3 INTRIGUE trial highlighted improved median progression-free survival in patients with KIT exon–mutated gastrointestinal stromal tumors compared with sunitinib.
Data from the phase 2 FIREFLY-1 trial indicated that tovorafenib yielded responses among pretreated patients with recurrent or progressive pediatric low-grade glioma.

Imetelstat demonstrated clinically meaningful and statistically significant improvement in transfusion independence at 24 weeks among patients with myelodysplastic syndrome in the phase 3 IMerge trial.

Among patients with metastatic pancreatic ductal adenocarcinoma, mitazalimab plus chemotherapy yielded a positive objective response rate in the phase 2 OPTIMIZE-1 trial.
The biologic license application for cosibelimab for patients with metastatic or locally advanced cutaneous squamous cell carcinoma is supported by findings from a phase 1 study.