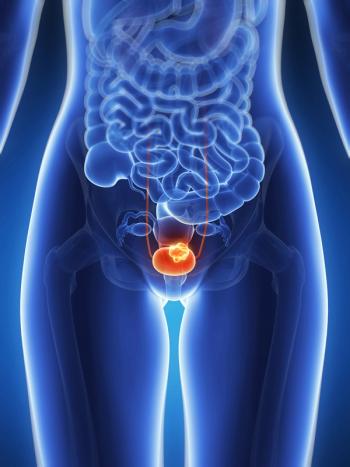
BT8009 monotherapy, which received fast track designation from the FDA, may be beneficial for adult patients with locally advanced or metastatic urothelial cancer.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

BT8009 monotherapy, which received fast track designation from the FDA, may be beneficial for adult patients with locally advanced or metastatic urothelial cancer.
The Japanese approval of acalabrutinib for the treatment of patients with treatment-naïve chronic lymphocytic leukemia was supported by data from the phase 3 ELEVATE-TN trial.
The biologic license application for toripalimab plus chemotherapy for the treatment of recurrent or metastatic nasopharyngeal carcinoma is expected to be resubmitted by mid-summer 2023.
The phase 3 NEPTUNE study indicated that durvalumab plus tremelimumab did not reach an amended primary end point of improved overall survival among patients with metastatic non–small cell lung cancer with a blood tumor mutational burden of at least 20 mut/Mb, though the combination did yield a numerical reduction in risk of death.
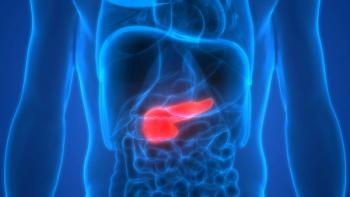
Data from the phase 1/2 CodeBreaK 100 trial indicated that 84% of patients with KRAS G12C-mutated pancreatic cancer treated with sotorasib experienced disease control.
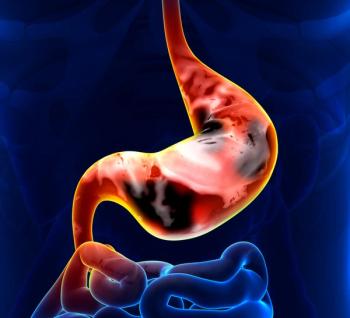
Topline results from the phase 3 GLOW trial indicated that zolbetuximab plus CAPOX yielded a statistically significant increase in progression-free survival over placebo/CAPOX.
The study’s lead author explained that using rezafungin may avoid some of the negative drug interactions associated with other anti-fungal drugs in patients with cancer.
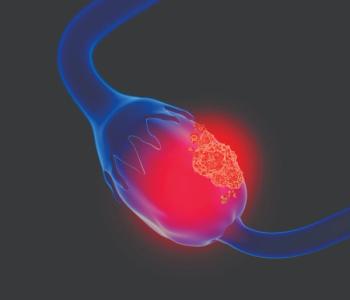
Findings from the phase 3 NORA study identified a numerically longer median overall survival among patients with platinum-sensitive recurrent ovarian cancer treated with maintenance niraparib regardless of BRCA mutation status.

Data from the phase 3 PEARL trial indicated that durvalumab yielded a clinically significant improvement in overall survival but did not meet statistical significance among patients with stage IV non–small cell lung cancer with high PD-L1 expression.
Olaparib in combination with abiraterone and prednisone or prednisolone alone has been approved as treatment for metastatic castration-resistant prostate cancer in the European Union.
The FDA granted breakthrough therapy designation to adagrasib plus cetuximab for patients with KRAS G12C–mutated advanced colorectal cancer based on findings from the phase 1b cohort of the KRYSTAL-1 trial.
The FDA has approved the FoundationOne Liquid CDx as a companion diagnostic for identifying patients with non-small cell lung cancer harboring EGFR mutations who may benefit from treatment with specific tyrosine kinase inhibitors.
The FDA has set the Prescription Drug User Fee Act date for April 21, 2023 for each supplemental biologics license application of enfortumab vedotin-ejfv and pembrolizumab in urothelial carcinoma.
The FDA has approved the Agilent Resolution ctDx FIRST assay for identifying patients with KRAS G12C–mutated non–small cell lung cancer who may benefit from adagrasib.

An expanded indication for pafolacianine as an imaging agent for patients with lung cancer has been approved by the FDA based on findings from the phase 3 ELUCIDATE trial.

The FDA has approved an additional indication for pemetrexed injection plus pembrolizumab and chemotherapy in the treatment of metastatic non–small cell lung cancer without EGFR or ALK tumor aberrations.
Findings from the phase 3 CONTACT-01 trial indicated that cabozantinib and atezolizumab did not reach the primary end point of overall survival in patients with metastatic non–small cell lung cancer.

Patients with KRAS G12C–mutated non–small cell lung cancer can now receive adagrasib therapy following the treatment’s recent accelerated approval by the FDA.
Findings from the phase 1b MajesTEC-2 trial indicated that teclistamab in combination with daratumumab and lenalidomide demonstrated potential for deep and durable responses in relapsed or refractory multiple myeloma.
Revumenib, which was given a breakthrough therapy designation by the FDA, may be beneficial in the management of relapsed or refractory KMT2A-rearranged acute leukemia based on data from the phase 1 AUGMENT-101 trial.
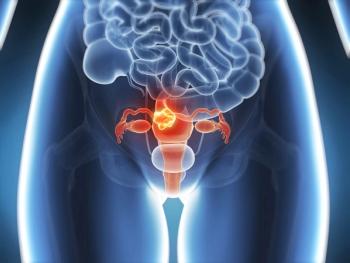
Treatment with dostarlimab plus chemotherapy demonstrated a statistically significant improvement in progression-free survival compared with placebo and chemotherapy among patients with advanced or recurrent endometrial cancer.
A secondary analysis from the phase 3 SWOG S1404 trial indicated that adjuvant pembrolizumab yielded improved patient-reported outcomes compared with high-dose interferon α or ipilimumab in the treatment of patients with high-risk resected melanoma.
The FDA’s acceptance of the biologics license application for denileukin diftitox for the treatment of patients with cutaneous persistent or recurrent T-cell lymphoma was supported by data from a pivotal phase 3 trial.

The FDA’s approval of oral olutasidenib provides adults with relapsed or refractory acute myeloid leukemia harboring a susceptible IDH1 mutation a new treatment option.

The FDA’s decision to grant fast track designation to batiraxcept for treating patients with advanced or metastatic clear cell renal cell carcinoma — a type of kidney cancer — was supported by data from a phase 1b trial.

Among patients with extramedullary multiple myeloma, a regimen consisting of daratumumab plus dexamethasone, cyclophosphamide, etoposide, and cisplatin yielded a complete remission rate of 35.5% and an overall response rate of 67.7%.

Rezvilutamide with androgen-deprivation therapy yielded improved overall survival and radiographic progression-free survival compared with bicalutamide and androgen-deprivation therapy in patients with high-volume metastatic hormone-sensitive prostate cancer.

Atezolizumab is no longer available to treat patients with advanced or metastatic bladder cancer following the manufacturer’s decision to withdraw its U.S. indication after consulting with the FDA.

Real-world patients with stage III colon cancer who were treated in the community practice setting with modified FOLFOX6 received less oxaliplatin vs historic controls from the phase 3 MOSAIC trial.
Results from a pooled case control study indicate that meninges are very radiosensitive in pediatric patients who were treated at prior to age 10 years, supporting reduced dose whole brain irradiation in this population.