
The addition of bortezomib to dexamethasone plus rituximab and cyclophosphamide appears to improve progression-free survival among those with Waldenström Macroglobulinemia.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

The addition of bortezomib to dexamethasone plus rituximab and cyclophosphamide appears to improve progression-free survival among those with Waldenström Macroglobulinemia.

Further research is necessary to determine whether regular DNA sequence testing for residual disease variants in patients with acute myeloid leukemia can improve outcomes.

Nirogacestat may be a noteworthy therapeutic advance for patients with desmoid tumors, according to an expert from Memorial Sloan Kettering Cancer Center.

Final analyses for pathologic response appear consistent with previous reports from the phase 3 AEGEAN trial evaluating neoadjuvant durvalumab and chemotherapy plus adjuvant durvalumab monotherapy in resectable non–small cell lung cancer.

The FDA initially issued a complete response letter for remestemcel-L in pediatric steroid-refractory acute graft-versus-host disease in October 2020, citing a need for an additional randomized, controlled trial to confirm the agent’s efficacy.

Findings from a multicenter analysis indicate that tumor lysis syndrome is a notable risk in patients with mantle cell lymphoma receiving treatment with venetoclax.

Abatacept plus ruxolitinib and screenings for concomitant respiratory muscle failure may be effective in reducing the rate of mortality related to immune checkpoint inhibitor–associated myocarditis among patients with cancer.
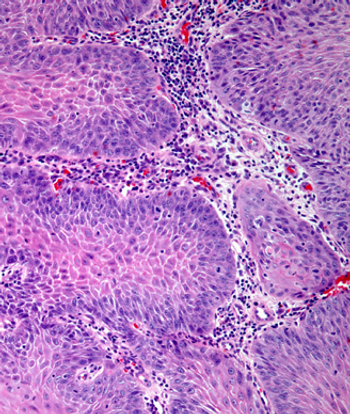
Camrelizumab plus apatinib demonstrates impressive anti-tumor activity in a pretreated population with recurrent or metastatic nasopharyngeal carcinoma, although further research is needed, according to an expert from Memorial Sloan Kettering Cancer Center.

The safety profile of fam-trastuzumab deruxtecan-nxki for managing HER2-expressing tumors in the DESTINY-PanTumor02 trial appears consistent with previously reported findings on the agent.

The VENTANA PD-L1 Assay becomes the only FDA-approved companion diagnostic with non–small cell lung cancer indications for 4 immunotherapy agents.

Pembrolizumab also appears to garner modest anti-tumor activity regardless of PD-L1 expression in patients with advanced thyroid cancer.

In particular, female patients who were considered obese with an advanced malignancy seem to have a higher incidence of high-grade immune-mediated adverse effects following treatment with nivolumab.

Lenvatinib and pembrolizumab’s benefit proves to be long-lasting and significant in first-line advanced renal cell carcinoma, according to an expert from University of Bari 'A. Moro'.

Pembrolizumab plus chemotherapy followed by resection and adjuvant pembrolizumab also appears to improve pathological responses in patients with non–small cell lung cancer.
Findings from a German register-based study suggests that more attention should be paid to sexual health among long-term hematological cancer survivors.
Adult patients with previously treated unresectable or metastatic HER2-positive breast cancer in China can now receive treatment with trastuzumab deruxtecan.

The European Medicines Agency also validates a type 2 variation marketing authorization application for nivolumab in resected stage IIB or IIC melanoma.
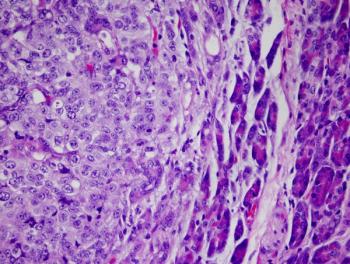
Previous research has identified a link between nutrients commonly found in a Mediterranean diet such as fiber, unsaturated fats, antioxidants, and polyphenols, and immunomodulatory/anti-tumor activities, according to a dietician from the University of Groningen.

Findings from the phase 3 DeFi trial support the new drug application for nirogacestat as a treatment for adults with desmoid tumors.
Findings from a retrospective cohort study suggest that circulating tumor DNA may be an important tool for risk-stratified treatment strategies in rhabdomyosarcoma.

Young adult survivors of childhood cancer may be at a higher risk of experiencing psychological and physical health issues associated with loneliness.
Adult patients with chronic lymphocytic leukemia in the European Union can now receive treatment with acalabrutinib tablets.

Data from the phase 2b KEYNOTE-942 trial support the FDA’s breakthrough therapy designation for adjuvant mRNA-4157-P201 plus pembrolizumab as treatment for high-risk resected melanoma.

The FDA and European Medicines Agency accept applications for elranatamab for relapsed or refractory multiple myeloma.

The FDA gives a positive recommendation following a Type C Meeting for the development of the OSE2101 cancer vaccine in advanced or metastatic non–small cell lung cancer.

A favorable Glasgow prognostic score appears to correlate with improved overall survival and progression-free survival following treatment with atezolizumab plus carboplatin and etoposide for small cell lung cancer.
An ongoing exploratory biomarker analysis of the CheckMate 9ER trial set out to identify predictive biomarkers or a composite model of response of nivolumab/cabozantinib efficacy in advanced renal cell carcinoma.
Prior exposure to recent bendamustine and having tumor-intrinsic features were associated with inferior efficacy outcomes following treatment with brexucabtagene autoleucel in relapsed or refractory mantle cell lymphoma.
Updated results from the phase 3 CheckMate 9ER trial continue to support nivolumab plus cabozantinib as a first-line treatment for patients with advanced or metastatic renal cell carcinoma.

Data from the European phase 1 MB-105 trial indicate that the safety profile of annamycin in geriatric advance acute myeloid leukemia are consistent with previously reported findings.