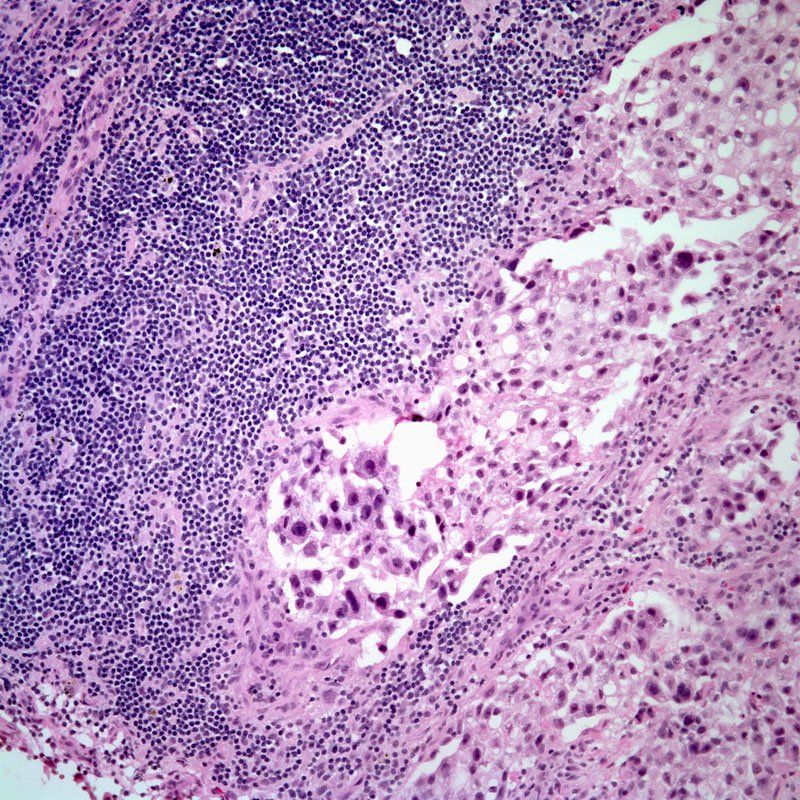
HER2-Positive Breast Cancer
Latest News
Latest Videos

CME Content
More News

The FDA granted a priority review to a new drug application submitted by Seattle Genetics, Inc. for tucatinib to treat patients with HER2-positive breast cancer.
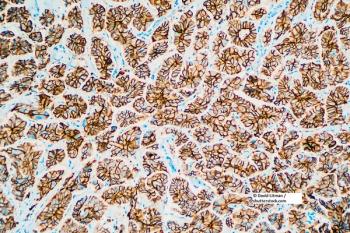
An analysis of the results of the phase III PANTHER trial showed that a combination of tailored dose-dense adjuvant chemotherapy and trastuzumab decreased the relative risk of relapse for patients with HER2-positive breast cancer.
The breast cancer expert spoke about the results presented in the trial, noting the benefit seen with longer follow-up.
The nurse practitioner from UCLA Health spoke about the expanding treatment options in this setting.

The FDA granted accelerated approval to trastuzumab deruxtecan for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer.

Seattle Genetics announced the development after data was presented at the 2019 San Antonio Breast Cancer Symposium.

The breast cancer expert discussed 2 advances in HER2-positive breast cancer treatment that healthcare providers should look for.

T-DXd demonstrated improved and durable response rates in heavily pretreated patients with advanced HER2-positive breast cancer.
The addition of pertuzumab to the previous standard of trastuzumab plus chemotherapy as an adjuvant therapy for patients with operable HER2-positive early breast cancer continued to reduce the risk for recurrence and death during a 6-year updated analysis.
Adjuvant therapy with S-1, an oral fluoropyrimidine-based drug, plus endocrine therapy postoperatively significantly increased invasive disease-free survival (IDFS) in patients with HR-positive, HER2-negative breast cancer.

The Food and Drug Administration approved the first generic for everolimus, which can provide a safe, effective, lower cost alternative to the brand-name drug it references.

Henlius announced that their multicenter phase III study met its primary end point of best overall response rate at week 24.

Oncotype DX® Breast Recurrence Scores from the National Cancer Database indicated that a lower threshold is needed for male patients with early stage ER-positive breast cancer to predict mortality.
PRS-343, a HER2/4-1BB targeting bispecific construct, is well-tolerated and demonstrated anti-tumor activity in heavily pre-treated patient population across multiple tumor types.
MCLA-128 showed radiological and clinical responses in patients with certain types of cancer who harbored neuregulin 1 gene fusions.
Treatment with trastuzumab emtansine demonstrated similar overall survival across 3 treatment arms of patients with HER2‐positive metastatic breast cancer.

Two trials and 3000 patients with HER2-positive breast cancer were analyzed for recurrence risk 5 to 10 years after diagnosis.

Tucatinib showed improvements in survival in patients with HER2-positive breast cancer.

Several clinicopathologic features of HER2-positive breast cancer patients were found to be associated with response to neoadjuvant therapy in a new study.
The trial examined HER2-positive breast cancer in patients with tumors smaller than 1cm; it is one of only a few to assess treatments in tumors this small.
A new study found a low rate of cancer recurrence at the nipple-areola complex (NAC) in patients with breast cancer who underwent nipple-sparing mastectomy (NSM).

A new combo may benefit patients with HER-2 positive metastatic breast cancer, according to a new study.

A new analysis looked at how dose interruptions or reductions of ado-trastuzumab emtansine in the treatment of advanced HER2-positive breast cancer impacted long-term survival outcomes.

A phase III trial tested trastuzumab emtansine (T-DM1) with or without pertuzumab in patients with HER2-positive metastatic breast cancer.

Researchers looked at the characteristics of breast cancer found in patients under 40 years old.