HER2-Positive Breast Cancer
Latest News

Latest Videos

CME Content
More News
A phase II trial tested docetaxel, trastuzumab, and pertuzumab vs T-DM1 for the neoadjuvant treatment of HER2+ breast cancer.

This phase III trial tested T-DM1 plus pertuzumab vs chemotherapy and dual HER2 blockade in patients with HER2+ breast cancer.
Researchers tested a novel antibody-drug conjugate known as trastuzumab deruxtecan in an expansion cohort of a phase I study of patients with advanced HER2+ breast cancer previously treated with trastuzumab emtansine.
Researchers tested whether certain patients with HER2-positive breast cancer may eventually be eligible for non-surgical management.

The long-term results of the phase III HannaH trial confirmed the similarity between the subcutaneous and intravenous formulation of trastuzumab in patients with HER2+ breast cancer.
Researchers tested omitting chemotherapy from a treatment regimen involving dual blockade with pertuzumab and trastuzumab in patients with metastatic HER2+ breast cancer.
The combination of trastuzumab and paclitaxel 'represents an important step forward in de-escalating therapy' for HER2+ breast cancer.
A genomic and proteomic analysis of HER2+ breast cancer cell lines that are resistant to trastuzumab found a deregulation of the cell death pathway known as TRAIL.
Researchers tested whether the use of alternative control probes to classify HER2 status in breast cancer could lead to substantial false positives.

The US Food and Drug Administration has approved the subcutaneous administration of trastuzumab for patients with HER2-overexpressing breast cancer.

An early-phase trial tested the combination of pembrolizumab with trastuzumab in patients with PD-L1–positive, trastuzumab-resistant, advanced HER2+ breast cancer.

The researchers looked at cardiac outcomes among a group of HER2+ breast cancer patients treated with trastuzumab who had an asymptomatic LVEF decline of < 50%.
The study findings suggest that disease-free survival can continue to be used as a surrogate endpoint in the setting of early HER2+ breast cancer.
Researchers tested whether combining trastuzumab/paclitaxel with dasatinib would have a high response rate in patients with metastatic HER2+ breast cancer.
The Opti-HER HEART trial examined whether liposomal formulations of chemotherapy agents may reduce cardiac toxicity in HER2+ breast cancer patients.

Dr. Charles Geyer discusses the phase III KATHERINE trial, which compared ado-trastuzumab emtansine vs trastuzumab in HER2-positive early breast cancer.
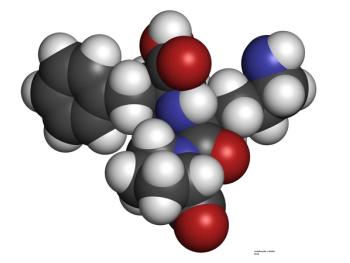
In patients with HER2-positive breast cancer treated with anthracyclines plus trastuzumab, can adding lisinopril or carvedilol reduce the risk of cardiotoxicity?

A comparison of chemotherapy regimens plus anti-HER2 therapy in the neoadjuvant setting tested whether the addition of anthracyclines is necessary for patients with HER2-positive breast cancer.

Researchers compared the cost effectiveness of two durations of trastuzumab in HER2+ breast cancer patients.

Dr. Adam Brufsky speaks with Cancer Network about the evolution and future of HER2-targeted therapy.

A subgroup analysis of Short-HER looked at the differences in 5-year DFS rates for 9 weeks vs 1 year of trastuzumab for HER2+ breast cancer patients.
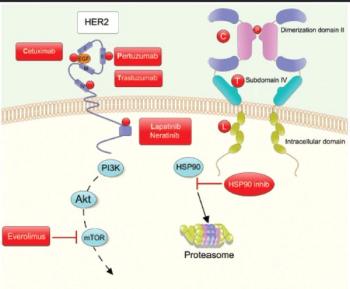
This article discusses the advances in HER2-targeted therapy, in both the metastatic and adjuvant settings, with a focus on those with early-stage breast cancer.
A novel cancer vaccine targeting HER2-positive malignancies has progressed quickly from mouse trials into early-phase human trials, with promising results.

The Short-HER study was unable to show noninferiority of 9 weeks of trastuzumab compared with the standard 1 year in women with HER2-positive breast cancer.

A new laboratory study has shed some light on how mitochondrial metabolism is altered in breast cancer.