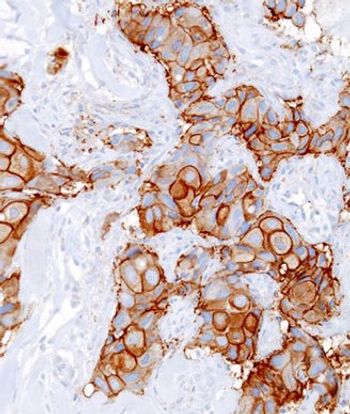
HER2-positive breast cancer patients treated with targeted therapy and chemo were less likely to have a pathologic complete response if they had a PIK3CA mutation.

Your AI-Trained Oncology Knowledge Connection!


HER2-positive breast cancer patients treated with targeted therapy and chemo were less likely to have a pathologic complete response if they had a PIK3CA mutation.
An 8-year follow-up of HER2-positive breast cancer patients receiving adjuvant trastuzumab shows an overall low incidence of cardiac events when administered after chemotherapy and radiotherapy.

After surgery, adjuvant therapy with trastuzumab alone works just as well as trastuzumab combined with lapatinib for women with early-stage HER2-positive breast cancer.
A follow-up analysis of gene expression signatures from the CALGB 40601 trial shows that certain HER2-positive early-stage breast cancer patients may not benefit from more aggressive chemotherapy treatments as part of a neoadjuvant regimen, and that patients with HER2-enriched tumors responded best to dual anti-HER2 treatment.

With the goal of helping to standardize and optimize care, ASCO has issued two new clinical guidelines on treating patients with HER2-positive breast cancer.

Dual HER2-targeted treatment with T-DM1 and pertuzumab resulted in positive antitumor activity in patients with HER2-positive, locally advanced or metastatic breast cancer, with an overall response rate of 41%.

It will be critically important to await the longer-term DFS and OS results from the neoadjuvant studies, as well as the adjuvant studies evaluating dual HER2 blockade, prior to these approaches truly becoming the standard of care.

Therapies targeting HER2 have revolutionized the treatment of breast cancer. Trastuzumab is the foundation of treatment for women with HER2-positive breast cancer. The challenge ahead is to develop predictors that can identify patients for whom trastuzumab alone will be sufficient.
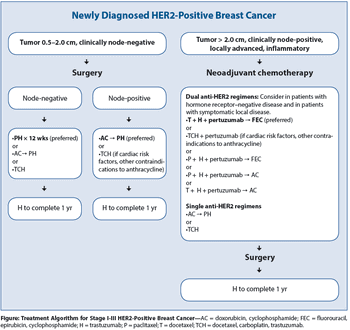
This review discusses the treatment of primary, nonmetastatic HER2-positive breast cancer in the adjuvant and neoadjuvant settings-settings in which tremendous progress has been made.
A phase I study of intermittent oral lapatinib in patients with HER2-amplified breast cancer escalated up to 7,000 mg per day (shown to be effective in mouse models), found that plasma concentrations of the drug did not increase proportionately with the oral dose, impeding clinical translation of this method.

Older age and comorbidities were associated with a higher risk of failing to complete trastuzumab therapy in a new study of older women with early-stage breast cancer, where nearly 20% failed to complete the treatment.

Contrary to some expectations, getting accelerated approval for neoadjuvant therapy does not look easy, and the pertuzumab story may be the exception that proves the rule.

At this point, there is expectation that pertuzumab given in the neoadjuvant setting will improve long-term efficacy. We welcome the opportunity to include pertuzumab in the neoadjuvant regimen of patients with HER2-positive breast cancer.
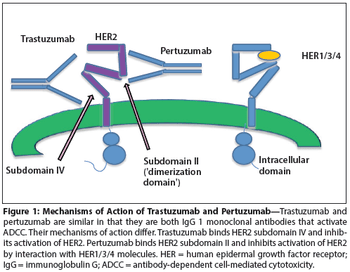
This article discusses the development of pertuzumab to date, with a particular focus on the accelerated approval decision. We highlight the need to identify reliable biomarkers of sensitivity and resistance to HER2-targeted therapy, which would make possible the individualization of treatment for patients with HER2-positive breast cancer.
Women who developed a rash early in their treatment for HER2-positive breast cancer with lapatinib were more likely to go on to have pathologic complete response compared to those who did not get a rash, according to the results of an unplanned analysis of data from the NeoALTTO trial.

Concurrent administration of trastuzumab and anthracyclines resulted in a similar rate of pathologic complete response when compared to sequential administration in women with HER2-positive breast cancer, according to the results of a new study.
Two studies indicate that older women with trastuzumab-treated breast cancer and those who undergo radiation to the left chest wall may be at risk for increased rates of cardiotoxicity.

New ASCO and CAP guidelines recommend HER2 testing for all newly diagnosed breast cancer patients with either early-stage invasive or metastatic disease.

T-DM1, a combination drug of trastuzumab and a cell-killing drug emtansine, significantly improved progression-free survival in women with advanced HER2-positive breast cancer whose cancer has recurred or progressed despite previous treatments, according to the results of the TH3RESA trial.

Long-term safety and efficacy results of two phase II trials indicate that dose-dense anthracycline-based chemotherapy with doxorubicin and cyclophosphamide can be safely combined with anti-HER2 therapy in women with early breast cancer.

The FDA today granted pertuzumab (Perjeta) accelerated approval for the treatment of patients with HER2-positive early stage breast cancer prior to surgery who are at high risk for recurrence or metastasis.

Combining four cycles of docetaxel and cyclophosphamide with 1 year of trastuzumab may be a viable treatment option for women with HER2-amplified early-stage breast cancer regardless of their TOP2A status, according to the results of a phase II study.

About 40% of women experienced a change in at least one biomarker from primary to residual breast cancer after undergoing neoadjuvant chemotherapy, according to the results of a study presented at the ASCO Breast Cancer Symposium.

Results of the PrefHer study indicated that when given the option between subcutaneous trastuzumab and intravenous trastuzumab, significantly more patients with HER2-positive breast cancer preferred the subcutaneous administration.

A retrospective analysis of the HERA trial indicated that young age was not associated with early recurrence in women with HER2-positive breast cancer, despite previous research suggesting that young age at diagnosis might be a risk factor for recurrence and death.