In the HERA trial, benefit for invasive lobular breast cancer patients with 1 year of trastuzumab maintenance was similar to invasive ductal carcinoma patients.

Your AI-Trained Oncology Knowledge Connection!


In the HERA trial, benefit for invasive lobular breast cancer patients with 1 year of trastuzumab maintenance was similar to invasive ductal carcinoma patients.
A study confirmed that PIK3CA mutations negatively affect survival in patients with HER2-positive breast cancer treated with the anti-HER2 therapy trastuzumab.
A new study shows that drugs targeting HER2 might provide good results in the rare non-small-cell lung cancer (NSCLC) patients with HER2 mutations.
Researchers participating in the phase III EMILIA trial have identified tumor biomarkers that can identify patients that are more likely to benefit from T-DM1. In the trial, women whose tumors had higher HER2 expression were most likely to benefit from the therapy.
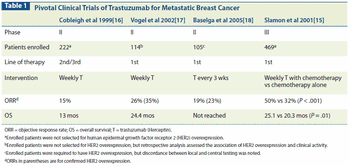
This article reviews clinical data informing the effective management of HER2-positive metastatic breast cancer, including the optimal sequence of HER2-targeted agents.

As more drugs become available in the HER2 arena, clinicians will be faced with increasing challenges regarding which sequences and combinations of drugs will be the best for their patients. In the era in which we practice, a great deal of this is likely to be dictated by the payers.

Third, how much do we really know about de novo and acquired resistance to trastuzumab and lapatinib? There are several possible clinical relevant mechanisms of trastuzumab resistance, including crosstalk with other receptors, amplification of the PI3K/AKT pathway, alteration of the trastuzumab binding domain, and loss of HER2 expression.

Last month brought the accelerated approval by the US Food and Drug Administration (FDA) of a fourth agent targeting the human epidermal growth factor receptor 2 (HER2) oncogene product: TDM-1 (Kadcyla), a conjugate of trastuzumab and a cytotoxic, emtansine.

A new study from cancer stem cell experts at the University of Michigan is challenging the notion that trastuzumab (Herceptin) only works for HER2-positive breast cancer.

Ado-trastuzumab emtansine, formerly known as T-DM1, improved progression-free survival as first-line treatment for patients with HER2-positive metastatic breast cancer, when compared with the standard treatment of trastuzumab plus docetaxel in a randomized phase II multicenter study.

As part of our coverage for the 30th Annual Miami Breast Cancer Conference, we bring you an interview with Dr. Mark Pegram, director of the breast cancer program at the Stanford Women’s Cancer Center and codirector of the molecular therapeutics program. Dr. Pegram will be discussing the potential for novel HER2 combination therapies at the conference.

The FDA has approved ado-trastuzumab emtansine (Kadcyla), known as T-DM1 in development, for the treatment of women with metastatic HER2-positive breast cancer.

Such a systematic review of the current status of mTOR inhibitors in the treatment of breast cancer demonstrates holes in our knowledge of the role of the tumor, the host, and metabolic factors in breast cancer progression.
Efforts to identify clinical biomarkers of response or resistance to mTOR inhibitors are ongoing. This review will summarize results of preclinical and clinical studies as well as ongoing clinical trials with mTOR or dual PI3K/mTOR inhibitors.

To kick off SABCS 2012, we discuss the use of molecular testing for the diagnosis and treatment of breast cancer patients in the clinical setting with Dr. Antonio Wolff of the Kimmel Cancer Center at Johns Hopkins University, one of the presenter's during the "Practical Use of Molecular Profiling" session at this year's symposium.

I see a time in the not too distant future when we’ll define tumors this way. What will our subspecialties be? Rather than a breast clinic or a lung clinic, will we perhaps be attending a “HER2 clinic” or an “mTOR clinic” instead?
This second article in our two-part series on targeted therapies in solid tumors covers the emergence of targeted therapies for the treatment of two common malignancies: lung cancer and breast cancer.
A new drug combination of lapatinib (Tykerb) and capecitabine (Xeloda) shrunk brain tumors in HER2-positive breast cancer patients whose cancer had spread to the brain, showing it is active as a first-line brain metastases treatment with similar efficacy to whole-brain radiotherapy.

We speak with Clifford Hudis, MD, Memorial Sloan-Kettering Cancer Center, about the recent advances in breast cancer treatment and the top news to come out of this year’s ASCO Breast Cancer Symposium.

According to a study of 12,500 women treated for invasive breast cancer, treatment with trastuzumab (Herceptin) increases the risk of congestive heart failure and cardiomyopathy.
Results of a phase III trial show early, promising data that the subcutaneous formulation of trastuzumab (Herceptin) is as efficacious and safe as the intravenous version of the drug.
The diagnosis of central nervous system (CNS) recurrence is a much dreaded outcome among breast cancer patients, and its incidence varies with disease stage and cancer subtype.

CancerNetwork speaks with Dr. Sara Hurvitz, director of the breast cancer program at the University of California in Los Angeles. Dr. Hurvitz is actively involved in translational phase I/II breast cancer clinical trials as well as in research to better define distinct types of breast tumors to better design novel targeted therapies.

CancerNetwork and the journal ONCOLOGY present an exclusive interview with Dr. Kimberly Blackwell, Duke Cancer Institute, who discusses some of the most important information to come out of this year’s meeting and talks about the future of breast cancer research.

Pertuzumab has been approved in combination with trastuzumab (Herceptin) and docetaxel chemotherapy for women whose breast cancer overexpress the HER2 receptor and who have not received prior systemic treatment.