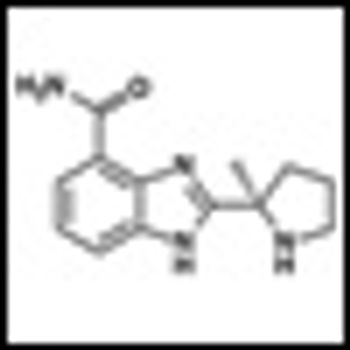
Normal B lymphocytes can follow either of two functionally distinct pathways of development. The first is a classical germinal center T-dependent pathway in which diversification and maturation generate a delayed but almost unlimited high-affinity response to antigens.