
Skin Cancer & Melanoma
Latest News
Latest Videos

More News

Data from RELATIVITY-047 show consistent benefits with nivolumab/relatlimab across most patient subgroups, including those with BRAF-mutated disease.

Nivolumab/Relatlimab Does Not Meet RFS End Point in Stage III/IV Melanoma
The safety profile of nivolumab plus relatlimab in patients with stage III/IV melanoma was consistent with the known profiles for each individual agent.

Neoadjuvant Pembrolizumab Appears Tolerable in Stage IIIB-D Melanoma
The phase 1/2 KEYMAKER-U02 trial evaluating pembrolizumab alone or as a combination therapy in stage IIIB-D melanoma found no adverse event–related deaths.
RP1 with nivolumab elicited an ORR of 38.7% with an acceptable safety profile in patients with advanced melanoma who progressed on anti–PD-1 therapy.
Investigators of the phase 3 C-POST trial will continue to follow up with patients and assess the key secondary end point of overall survival.

Nivolumab/Ipilimumab Continues to Show Survival Benefit in Melanoma
The CheckMate 067 trial found that, at a 10-year follow-up, nivolumab/ipilimumab elicited a median OS of 71.9 months in patients with previously untreated, advanced melanoma.
According to Jason Luke, MD, FACP, there is still room to improve response rates in CSCC treatment, but there is excitement surrounding future of treatment outcomes.

Objective response data from the CK-301-101 trial supports cosibelimab’s approval by the FDA, according to Jason J. Luke, MD, FACP.

Cosibelimab has been approved by the FDA as a treatment for patients with cutaneous squamous cell carcinoma.

Treatment Decisions in Metastatic Melanoma: Immunotherapy vs Targeted Therapy
Panelists discuss how treatment decisions in metastatic melanoma involve balancing the benefits of immunotherapy versus targeted therapy, taking into account factors such as tumor genetics, patient response, and tolerability.

Vusolimogene oderparepvec in combination with nivolumab has received breakthrough therapy designation from the FDA in advanced melanoma.

Phase 1 data highlight a manageable safety profile with IMA203 among patients with melanoma and other PRAME-positive solid tumors.
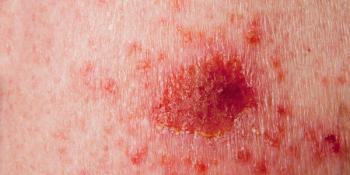
Phase 3 data may support the photodynamic therapy as a noninvasive treatment option for patients with superficial basal cell carcinoma.
Phase 1b findings may affirm the therapeutic potential of IMA203 for patients with previously treated metastatic melanoma.
The safety profile of fianlimab/cemiplimab in a phase 1 trial was consistent with prior reports of cemiplimab monotherapy.

Phase 2 data also show responses with atezolizumab switch therapy in a population of patients with BRAF V600–positive melanoma.

Pre-BLA Meeting Successful for RP1 Combo in Anti–PD-1–Failed Melanoma
Support for the planned biologics license application comes from data showing a 32.9% response rate with RP1 plus nivolumab for melanoma.

Support for the decision follows phase 1 findings evaluating IBI363 in patients with advanced solid tumors presented at the 2024 ESMO Plenary.
Preliminary results from part 2 of the phase 2 trial evaluating VP-315 in basal cell carcinoma found no dose-limiting toxicities or treatment-related serious adverse events.

The Melanoma Research Alliance mourns the passing of Jeffrey S. Weber, MD, PhD, a pioneer in developing immunotherapy for patients with melanoma.

Data from the phase 3 MELATORCH study support the supplemental new drug application for frontline toripalimab in unresectable or metastatic melanoma.

Treatment Practices in Metastatic Melanoma
Allison Betof Warner, MD, PhD, and the Oncology Brothers share clinical insights on treatment decisions for patients with metastatic melanoma.

Neoadjuvant Therapy in Melanoma
Medical oncologists provide comprehensive insights on neoadjuvant therapy options for patients with melanoma.

Treatment Algorithm for Stage IIB and Beyond Melanoma
Allison Betof Warner, MD, PhD, joins Rahul Gosain, MD, and Rohit Gosain, MD, to discuss treatment practices for patients with stage IIB and beyond melanoma.
New guidelines for both cancer types provide detailed and distinct criteria for diagnoses, staging, use of IGRST, and follow up, among other areas.




























































