Skin Cancer & Melanoma
Latest News
Latest Videos

CME Content
More News
Imaging after 1 week of treatment with pembrolizumab may help identify treatment responses in patients with metastatic melanoma.

Patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody can now receive lifileucel after accelerated approval from the FDA.
The DermaSensor device demonstrates a high rate of sensitivity in the detection of more than 200 types of skin cancers in a clinical study.

Results from a long-term analysis of the phase 3 IMCgp100-202 trial indicate that tebentafusp results in better disease control and long-lasting responses in those with HLA-A*02:01–positive, previously untreated metastatic uveal melanoma.

mRNA-4157 is being evaluated in combination with pembrolizumab as part of the phase 2b KEYNOTE-942/mRNA-4157-P201 trial in patients with completely resected, high-risk, stage III to IV melanoma.

Treatment with mRNA-4157 plus pembrolizumab yields benefits in resected melanoma subgroups in the phase 2 KEYNOTE-942 trial, including those with BRAF-mutated tumors.

Those with advanced mucosal melanoma who were treated with lifileucel saw clinically meaningful activity.

Results presented at 2023 ESMO showed a prolonged survival benefit in patients receiving adjuvant cemiplimab for stage II-IV cutaneous squamous cell carcinoma.

Nivolumab is now available as an adjuvant treatment for patients with completely resected stage IIB or IIC melanoma following its approval by the FDA based on data from the phase 3 CheckMate-76k trial.

Second-line end points of the TRICOTEL trial assessing atezolizumab plus vemurafenib and cobimetinib in melanoma with central nervous system metastases appear consistent with primary efficacy end points.
Data from the phase 3 CheckMate-76k trial support the European Commission’s approval of nivolumab as an adjuvant treatment for patients with resected stage IIB or IIC melanoma.

Supporting data for the FDA approval of melphalan administered via percutaneous hepatic perfusion for metastatic uveal melanoma come from the phase 3 FOCUS study.

Using the 31-gene expression profile assay may help lead to more personalized treatment strategies in patients with cutaneous melanoma, according to Aaron Farberg, MD.
Investigators of a phase 1 study evaluating cosibelimab in patients with locally advanced or metastatic cutaneous squamous cell carcinoma plan to share updated results at a future medical conference.

The benefit of nivolumab/ipilimumab plus single-agent nivolumab for unresectable advanced melanoma with poor prognostic characteristics was comparable in all-comers and patients with brain metastases.

Combination therapy with nivolumab and relatlimab produces superior progression-free survival vs nivolumab monotherapy among those with advanced melanoma in the phase 3 RELATIVITY-047 trial.
mRNA-4157 plus pembrolizumab appears well tolerated without an increase in immune-mediated adverse effects vs pembrolizumab monotherapy among those with high-risk melanoma in the phase 2 KEYNOTE-942 trial.

Targeting apoptosis in BRAF V600-mutated melanoma may be considered for future exploration in the post immunotherapy setting, according to an expert at Moffitt Cancer Center.

The safety of fianlimab plus cemiplimab appears consistent with the safety profile previously observed with each individual agent among patients with previously treated metastatic melanoma in a phase 1 study.
Common treatment-emergent adverse effects among patients receiving PRT811 for uveal melanoma or advanced glioma include nausea and vomiting in a phase 1 study.

The FDA sets a Prescription Drug User Fee Act date of November 25, 2023 for lifileucel in the treatment of patients with advanced melanoma.
Treatment with isolated hepatic perfusion appears to yield improved progression-free survival compared with best alternative care in patients with isolated uveal melanoma liver metastases.
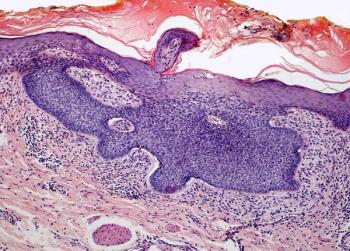
The 31-gene expression profile test appears to allow for significant and independent risk stratification of those with stage I cutaneous melanoma.

Tavokinogene telseplasmid plus pembrolizumab is reported to be well tolerated in patients with advanced, pre-treated melanoma based on data from the phase 2 KEYNOTE-695 trial.

Patients with metastatic or recurrent locally advanced Merkel cell carcinoma can now receive treatment with retifanlimab-dlwr following its accelerated approval by the FDA.