
The FDA has approved nivolumab for patients with high-risk urothelial carcinoma regardless of previous treatment with neoadjuvant chemotherapy, nodal involvement, or PD-L1 status.

Your AI-Trained Oncology Knowledge Connection!


The FDA has approved nivolumab for patients with high-risk urothelial carcinoma regardless of previous treatment with neoadjuvant chemotherapy, nodal involvement, or PD-L1 status.

FT596 and FT516 natural killer cell products elicited promising responses in a population of patients with B-cell lymphoma.
Findings from the phase 2 DART trial demonstrated that the combination of ipilimumab and nivolumab may have potential for the treatment of patients with angiosarcoma.

Black patients with non–small cell lung cancer were more likely to be diagnosed at advanced stages of disease, less likely to receive surgery in early stages, and had increased cancer mortality rates than White patients.
Patients with solid tumors experienced significant immunogenicity within 2 weeks of receiving a second dose of the BNT162b2 COVID-19 vaccine.

The phase ORIENT-16 trial indicated that the addition of sintilimab to chemotherapy significantly improved of overall survival compared with placebo.

The VENTANA MMR RxDx Panel is the first FDA-approved companion diagnostic for identifying patients with DNA mismatch repair–deficient solid tumors who might benefit from treatment with dostarlimab-gxly.
Investigators theorize that a filgrastim-sndz biosimilar is an affordable option for patients across a number of tumor types who are undergoing curative intent treatment with chemotherapy and have an intermediate risk for experiencing febrile neutropenia.

A genomic-adjusted radiation dose model can aid in predicting radiotherapy benefit vs physical dose of radiotherapy pan-cancer.

A pooled analysis of 3 randomized trials highlight the potential benefit of utilizing androgen receptor inhibitors in men aged 80 years or older with non-metastatic castration-resistant prostate cancer.
The prospective observational Precision1 cohort study will integrate imaging technology with actionable whole genome sequencing in patients with primary and secondary liver cancer.

The folate receptor α–targeting antibody-drug conjugate STRO-002 has a fast track designation from the FDA for advanced, platinum-resistant ovarian cancer following 1 to 3 prior lines of therapy.
Gut microbes may help to predict adverse effects and outcomes in patients with advanced melanoma who are being treated with dual immune checkpoint inhibitors.

The World Health Organization has published new recommendations to aid in the global prevention of cervical cancer through more accessible screenings and treatments.

Solid tumors with mismatch repair deficiency that have progressed on or after prior treatment and which have no suitable alternative therapy options may now be treated with dostarlimab following its approval by the FDA.
Research on NCCN resource-stratified guidelines for treating breast cancer could lead to overtreating patients in lower-middle and upper-middle income countries, while low-income populations could receive undertreatment.

New findings indicate that an increased intake of vitamin D may help to prevent the development of early-onset colorectal cancer in individuals under the age of 50.

Patients with recurrent pancreatic cancer could potentially benefit from stereotactic body radiotherapy plus pembrolizumab and trametinib post-surgery.
Patients with chronic lymphocytic leukemia/small lymphocytic lymphoma were pretreated with ibrutinib saw a reduction in obinutuzumab-induced infusion-related reactions as well as cytokines and chemokines.
At a population level, those who were selected for surgery had a greater risk of dying from cancer related causes than dying from non-cancer causes.

Investigators advise against the use metformin in addition to chemoradiotherapy for patients with locally advanced non–small cell lung cancer due to progression-free survival and overall survival was not substantial.

The FDA has granted IN10018 a fast track designation for the treatment of patients with platinum-resistant ovarian cancer.

CancerNetwork’s latest podcast episode features a conversation with Anath Ravi, PhD, of MOLLI Surgical and Randy Miles, MD, MPH, of Massachusetts General Hospital on the risks and benefits of breast cancer screening for average-risk women under the age of 50 years.
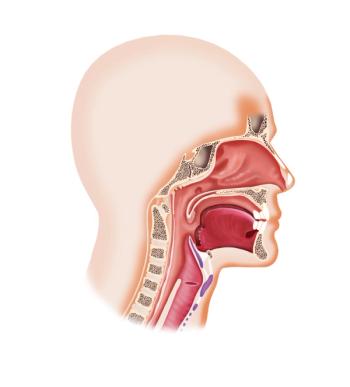
Investigators identified an improvement in median progression-free survival among patients with recurrent or metastatic nasopharyngeal carcinoma who were treated with camrelizumab plus gemcitabine and cisplatin vs placebo plus gemcitabine.

Transgender individuals may be more likely to develop breast cancer than cisgender men; guidelines need to be established and institutions need to eliminate discrimination to encourage higher breast cancer screening rates for this population of patients, investigators said.

Toripalimab in combination with chemotherapy yielded an improvement in progression-free survival compared with placebo plus chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma.
Although most patients with cancer who received both doses of the COVID-19 vaccine developed a good immune response within the following 3 weeks, investigators emphasized the importance of developing alternative strategies for patients who are at a high risk of not responding.

The use of belzutifan has been approved by the FDA for patients with cancer that is associated with von Hippel-Lindau disease who do not need immediate surgery.

The results from a phase 1 trial that focused on a second cohort of patients with unresectable or metastatic melanoma identified positive safety and promising topline survival outcomes when treated with the combination of UV1 and pembrolizumab.

Although healthy patients do not currently require a third dose of the vaccine, the FDA has authorized the use of an additional dose of the Pfizer or Moderna COVID-19 vaccines for individuals who are immunocompromised.