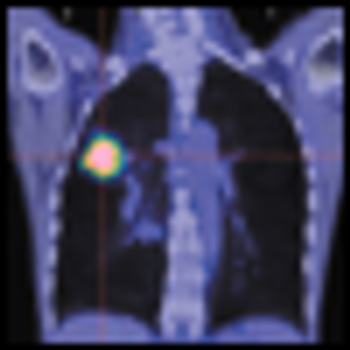
It is well known that exposure to chemotherapy or radiation therapy can result in long-term complications for childhood cancer survivors. What is less certain is why some children have to contend with these complications while others do not. Researchers at the City of Hope Medical Center in Duarte, Calif., are one step closer to fitting another piece in the survivorship puzzle: They hypothesized that there is some inherent genetic susceptibility that raises this risk.