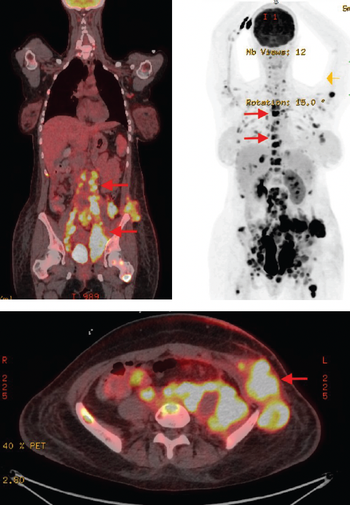
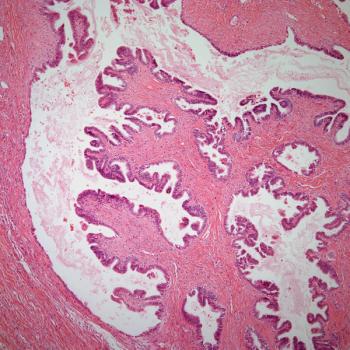
Discrepancies in patients with blood cancer who are eligible for but do not undergo hematopoietic stem cell transplant have been observed not only for Black patients, but Hispanic, Asian, and White patients as well, according to Usama Gergis, MD, MBA.