The hospitalist at Memorial Sloan Kettering Cancer Center discussed the results of a study which evaluated the use of 177Lu-DOTATATE in patients with well-differentiated, high-grade neuroendocrine tumors.

Your AI-Trained Oncology Knowledge Connection!


The hospitalist at Memorial Sloan Kettering Cancer Center discussed the results of a study which evaluated the use of 177Lu-DOTATATE in patients with well-differentiated, high-grade neuroendocrine tumors.

The expert in hematology/oncology discussed the results of a study which evaluated the use of peptide receptor radionuclide therapy in patients with well-differentiated neuroendocrine tumors.
Data published in JAMA Oncology found boosts in quality of life in addition to improvements in depression, anxiety, and posttraumatic stress symptoms for patients with acute myeloid leukemia receiving integrated palliative and oncology care.
Overall, the phase 3 ELEVATE-RR trial demonstrated efficacy of acalabrutinib and a safety and tolerability profile that with was consistent with that observed in the broader acalabrutinib clinical development program.
Expert details the potential for a clinical trial using minimal residual disease to guide therapy for patients with DLBCL.
Just over a month after the FDA issued Emergency Use Authorization to the first mRNA vaccine for COVID-19, the National Comprehensive Cancer Network released initial recommendations for its use in patients with cancer.
Investigators suggested that the results of this study may assist health care providers in personalized therapeutic regimen selections for patients with early-stage breast cancer.

“We’re hearing about other antibody-drug conjugates, other agents in hormone receptor–positive metastatic disease, and the next generation of drugs that we’ll be using to treat our patients.”
Merryman explains the value of evaluating separate transplant-related questions for patients with relapsed or refractory diffuse large B-cell lymphoma.
Imagio, a diagnostic tool that uses novel technology to provide real-time information on suspicious lesions in the breast, is OK’d by the FDA.
Data published in Cancer the journal found that the response to neoadjuvant therapy in patients with retroperitoneal sarcoma following surgery was fair overall, with data suggesting the potential for disease progression as a predictive tool for patient survival.
The expert in multiple myeloma spoke about the research that she believes will be most influential for patients with multiple myeloma.

The FDA granted approval to the combination use of cabozantinib plus nivolumab ahead of its February PDUFA date as therapy for the first-line treatment of patients with advanced renal cell carcinoma.
Findings from a phase 1/2 trial indicate that lisocabtagene maraleucel plus ibrutinib may represent a viable treatment option for patients with heavily pretreated chronic lymphocytic leukemia or small lymphocytic lymphoma and high-risk disease features.
Research published in JAMA Network Open found that total neoadjuvant therapy enhanced pathological complete response rates for patients with locally advanced rectal cancer.

The BLA submission is based on data observed in the phase 2 POD1UM-202 trial of retifanlimab in previously treated patients with locally advanced or metastatic squamous cell carcinoma of the anal canal who had progressed on, or were intolerant of, standard platinum-based chemotherapy.

The novel viral immunotherapy PVSRIPO is designed to stimulate a patient’s innate and adaptive immune system to promote an antitumor response and establish long-term immunologic memory to help keep their cancer at bay.
“Our results suggest that low [intertumoral heterogeneity] is associated with increased response to anti–PD-1 immunotherapy in renal cell carcinoma through increased immune activity involving more neoantigens and less frequent immune evasion,” wrote the study authors, led by Xia Ran.
The leukemia expert talks about how current findings may hold promise for a larger group of patients in the future.
Compared with previous standard-of-care therapy with sorafenib, the IMbrave150 combination maintains overall survival superiority in treatment-naive advanced hepatocellular carcinoma.
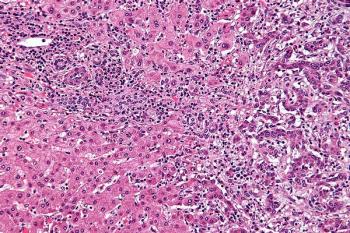
Encouraging efficacy and safety outcomes were reported at the 2021 Gastrointestinal Cancers Symposium when infigratinib was used to treat FGFR2 fusion–positive cholangiocarcinomas that are refractory to chemotherapy.

Thought leader detailed the findings from an oral presentation investigating patients with relapsed or refractory diffuse large B-cell lymphoma.

Overall survival benefits were noted when ivosidenib tablets were used in previously treated patients with IDH1-mutant cholangiocarcinoma when compared against placebo, according to a phase 3 trial.
The expert in multiple myeloma highlighted the research which she was most excited to see presented at the ASH Annual Meeting & Exposition.

The Breast Cancer Index assay is the only of its kind to be recommended in the National Comprehensive Cancer Network Guidelines for the treatment of breast cancer as being predictive of extended adjuvant endocrine therapy.

The ongoing phase 3 CASPIAN trial evaluating the use of intravenous durvalumab, with or without tremelimumab, added to platinum–etoposide for the first-line treatment of extensive-stage small cell lung cancer demonstrated improved overall survival versus chemotherapy alone.

Investigators aimed to evaluate additional end points from the phase 3 RxPONDER trial in women with hormone receptor–positive, HER2-negative, lymph node–positive breast cancer.

Bristol Myers Squibb announced that nivolumab has been granted priority review for indications in both the first-line and adjuvant treatment settings as therapy for gastrointestinal cancers.

The assistant professor of Urology at The University of Texas MD Anderson Cancer Center spoke about the results of a study which evaluated the use of the Mediterranean diet in men with localized prostate cancer on active surveillance.
No significant benefits to progression-free survival were noted with the axitinib-plus-octreotide combination for the treatment of patients with advanced G1-2 extra-pancreatic neuroendocrine tumors.