
The Federal Trade Commission has charged four US cancer charities with fraud, accusing them of diverting more than $187 million in charitable donations.

Your AI-Trained Oncology Knowledge Connection!


The Federal Trade Commission has charged four US cancer charities with fraud, accusing them of diverting more than $187 million in charitable donations.
Patients with acute myeloid leukemia who had a DNMT3A mutation had poorer disease prognosis, according to an analysis of patients aged 60 years or younger.
A Swedish study found that 3D tomosynthesis screening detected 40% more cases of breast cancer compared with traditional digital mammography.

A prospective study found that a bronchial airway gene-expression classifier improved the diagnostic performance of bronchoscopy for lung cancer detection.

The appearance of bone metastases on FDG PET/CT was influenced by breast cancer histology, with non-FDG-avid lesions more common in invasive lobular carcinoma patients.

Genotyping of African Americans suggest that with regard to somatic driver mutations in non-small-cell lung cancer (NSCLC), black patients do not significantly differ from white patients, according to a new study.

In a mouse model, researchers found that TRAIL-R2 inhibition prevented bone metastases, suggesting a possible therapeutic strategy for breast cancer patients.
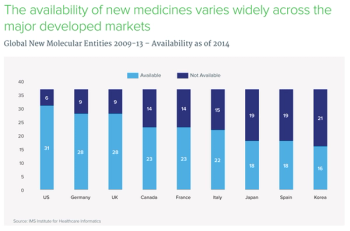
This slide show looks at patient access to oncology agents both in the United States and abroad, and examines barriers and other constraints preventing new treatments from getting into the hands of patients.

Advocacy is about making sure that our lawmakers enact the best healthcare policies for our patients. Just as we have a duty to our patients in the clinic, we also have a duty to advocate for laws that benefit patients’ health.

As part of our coverage of the 2015 ONS Annual Congress, we discuss genetic testing for cancer patients and the role of oncology nurses.
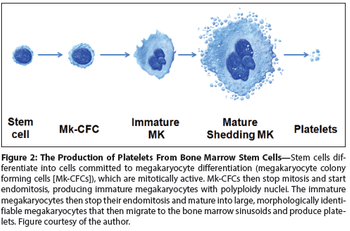
This review will focus on the general approach to, and treatment of, thrombocytopenia in cancer patients, including thrombopoietin treatment in patients receiving non-myeloablative chemotherapy.

In a recently published study, patients who did not respond to initial radiation or re-irradiation of symptomatic bone metastases had significantly higher urinary markers of bone turnover at baseline and follow-up.

A recent retrospective study elucidates the correlation between breast cancer subtype and metastasis site, time to relapse, and patient survival.

A trite modern metaphor for the absence of good options refers to those choices that are only “better than a sharp stick in the eye.” I was recently faced with a medical problem where I had to decide whether I had an option superior to a sharp stick in the eye.

A new study has shown that consumption of fish oil can raise the plasma levels of fatty acids, which may cause resistance to chemotherapy for cancer treatment.
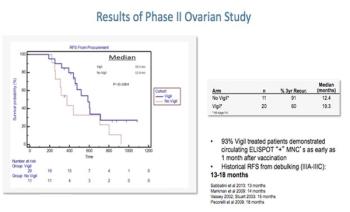
This slide show features some of the top highlights from the 2015 Society of Gynecologic Oncology Annual Meeting, including a vaccine trial in ovarian cancer and a possible biomarker in advanced cervical cancer.
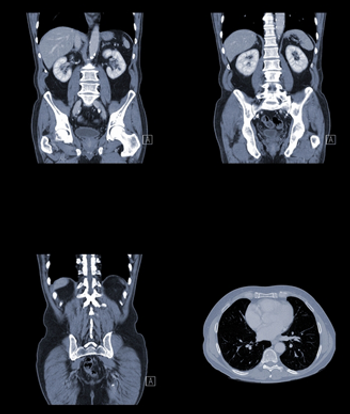
Use of routine follow-up imaging to monitor asymptomatic diffuse large B-cell lymphoma patients resulted in a small survival benefit, but at a substantial cost.

In this interview we discuss the recent CMS decision to cover low-dose CT screening for lung cancer in patients who fit specific criteria.

The risk of bone metastases from GISTs, though rare, should be considered during long-term follow-up of patients, especially in those with liver metastases.
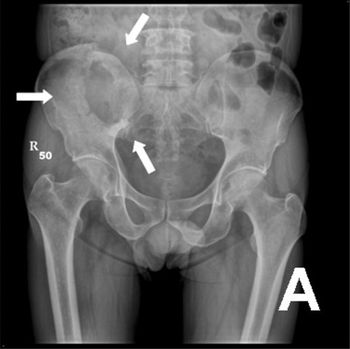
Researchers were able to demonstrate response to radiotherapy in breast cancer patients with osteolytic metastases by measuring increases in bone density.
Accumulating evidence suggests that aspirin could decrease the risk of a cancer diagnosis or the chance of death from commonly diagnosed cancers. However, studies and clinical trials have been mixed, and do the benefits outweigh the risks of gastrointestinal bleeding and peptic ulcers?
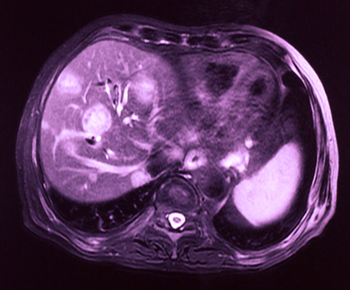
Current use of statins was associated with about a 50% decreased risk for liver cancer, according to results of a study looking at data from the United Kingdom.

A study found varying degrees of costs and benefits associated with granting early access to drugs based on PFS. In many cases, costs outstripped the benefits.

More and more cancer patients are surviving their diseases as better treatments become available, and while that’s good news, it poses challenges for clinicians.

A state of equipoise now exists for various surgical options in the treatment of early lung cancer, underscoring the need for shared decision making.

Smaller early-stage tumors may lend themselves to less radical lung parenchymal sparing resections or no surgery at all.

This review looks at the current data and guidelines for thoracoscopic resection of stage I NSCLC and discusses the potential for limited lung resection in patients with the disease.

Although genomic testing can improve the cost-effectiveness of a treatment, assessing the cost-effectiveness of genomic testing outside the context of its impact on treatment is not practical.

The identification and characterization of gene signatures, driver events, and pharmacogenomics in molecularly homogeneous subsets of patients is likely to advance effective drug development strategies in colorectal cancer.

Numerous genomic tests are available for use in colorectal cancer, with a widely variable evidence base for their effectiveness and cost-effectiveness. In this review, we highlight many of these tests, with a focus on their proposed role, the evidence base to support that role, and the associated costs and risks.