
Numerous clinicians spoke about breaking news, clinical trial updates, and the evolution of standard-of-care treatment throughout 2025.

Your AI-Trained Oncology Knowledge Connection!


Numerous clinicians spoke about breaking news, clinical trial updates, and the evolution of standard-of-care treatment throughout 2025.

Based on results from the MATTERHORN study of durvalumab/FLOT in gastric/GEJ cancers, the regimen should be considered a new SOC.

Michelle Riba, MD, discusses the evolution of distress screening in oncology and the move toward a collaborative care model to integrate psychosocial support into clinical practice.

From KRAS inhibitors in metastatic CRC to radioligands and PET imaging agents in prostate cancer, dozens of oncologic approvals were reported in 2025.

Yelena Y. Janjigian, MD, provides the background of the MATTERHORN trial, showcasing the importance of durvalumab plus FLOT in gastric/GEJ cancers.

Daniel C. McFarland, DO; and Charles Kamen, PhD, MPH, discuss the unique challenges that sexual and gender minority groups experience in cancer care.

Moving the needle in cancer research was an important focus for the journal ONCOLOGY in 2025.

Throughout 2025, our podcast highlighted experts who discussed the latest conference data, newly approved drugs, and other oncologic happenings.
Treatment with PT-112 appeared to be well-tolerated among those with recurrent thymoma in a phase 2 trial.

Among 35 patients with ovarian cancer treated with an antibody-based combination, the overall response rate was 23%, with a clinical benefit rate of 31%.
Safety data from ASCENT-03 support sacituzumab govitecan as an effective therapy with manageable toxicities in advanced triple-negative breast cancer.

Kandance P. McGuire, MD, FACS, breaks down what lymphedema is and why it may be detected later.
Data from the phase 3 MANUEVER trial support the approval of pimicotinib for patients in China with symptomatic tenosynovial giant cell tumors.
Results from arms C and D of the phase 3 SEQUOIA trial demonstrated that zanubrutinib alone or in combination with venetoclax yields positive results in CLL/SLL subpopulations.
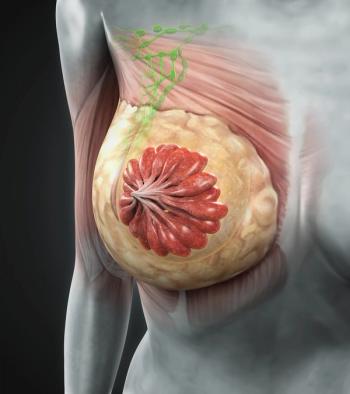
Exploratory data from a phase 3 study may help optimize adjuvant endocrine therapy choices in hormone receptor–positive, HER2-positive breast cancer.

A total of 33% of patients with advanced prostate cancer receiving the combination therapy remained progression-free per PSA after 1 year of treatment.

Treatment with ceralasertib plus durvalumab was found to be well tolerated among patients enrolled on the phase 3 LATIFY trial.

How John G. Phillips, MD, MPH, and Tennessee Oncology are aiming to expand radiation oncology access in smaller communities across Tennessee.
C. Ola Landgren, MD, PhD, and Noopur Raje, MD, discussed results from the ADVANCE trial of DKRd in patients with newly diagnosed multiple myeloma.
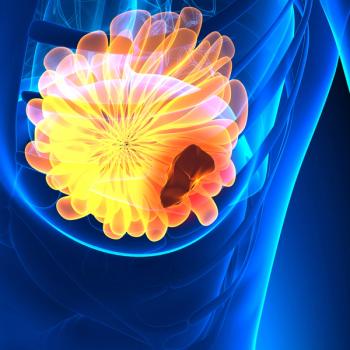
Results from the DESTINY-Breast05 trial revealed that T-DXd was superior to T-DM1 in these patients with HER2-positive early breast cancer.

Data from the phase 1/2 GO29781 study support the approval of subcutaneous mosunetuzumab in this follicular lymphoma population.
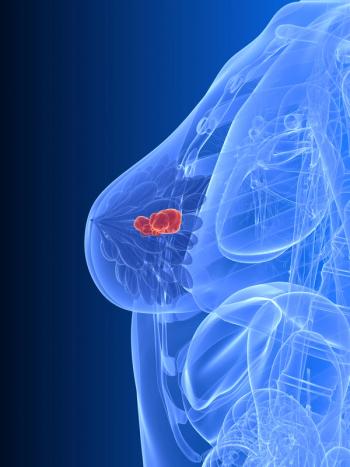
Unadjusted post-recurrence OS was numerically lower with palbociclib plus endocrine therapy vs endocrine therapy alone in the phase 3 PALLAS trial.

Daniel C. McFarland, DO; and Michelle B. Riba, MD, spoke about distress screening and integrating psychosocial care into oncology.

Pooled analysis data from the phase 1 JSKN003-101 and phase 1/2 JSKN003-102 trials support the regulatory decision.

“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.
Data from the phase 3 PHILA study support pyrotinib plus trastuzumab as a therapeutic strategy in HER2-positive metastatic breast cancer.
Data from the PREFER study may provide evidence for improving oncofertility counseling for premenopausal women with early breast cancer.
“This trial supports the value of a functional RAD51-based HRD assessment to refine patient selection for PARP inhibitors,” said Isabel Pimentel, MD.
The risk of pain deterioration was similar with T-DXd/pertuzumab vs standard of care in the DESTINY-Breast09 trial.
Data from a phase 1 trial may support additional clinical studies of CT0596 in relapsed/refractory multiple myeloma.