
Eflornithine, which was approved in adult and pediatric patients with high-risk neuroblastoma, was assessed in an externally controlled trial comparing results from Study 3b and Study ANBL0032.

Your AI-Trained Oncology Knowledge Connection!


Hayley Virgil is a senior editor with CancerNetwork. When she isn't traveling to conferences and championing health equity in the oncology space, she can be found hiking, foraging wild plants, gardening, sewing ballgowns, practicing embroidery, or playing video games.

Eflornithine, which was approved in adult and pediatric patients with high-risk neuroblastoma, was assessed in an externally controlled trial comparing results from Study 3b and Study ANBL0032.

The European approval for dostarlimab and chemotherapy for dMMR/MSI-H, primary advanced or recurrent endometrial cancer is based on data from part 1 of the phase 3 RUBY/ENGOT-EN6/GOG3031/NSGO trial.

Investigators report that response-adapted trials utilizing novel combination regimens appear to be safe and feasible in a population of patients with newly diagnosed diffuse large B-cell lymphoma.

Investigators report no new safety signals in patients with relapsed/refractory follicular lymphoma following treatment with tisagenlecleucel infusion.

Patients with chronic lymphocytic leukemia and small lymphocytic lymphoma can now receive treatment with pirtobrutinib.

Second-line treatment with belantamab mafodotin plus bortezomib/dexamethasone in patients with relapsed/refractory multiple myeloma is supported by findings from the phase 3 DREAMM-7 study.

Investigators report a trend in overall survival improvement among patients with advanced/recurrent endometrial cancer treated with atezolizumab and chemotherapy.
Data from the phase 3 EV-301 study turn up no new safety signals in patients with advanced urothelial carcinoma treated with enfortumab vedotin.

In the October edition of Snap Recap, we review the latest FDA news and the vote from the last ODAC meeting.

Progression-free survival benefit appears consistent in patients with recurrent or metastatic cervical cancer treated with tisotumab vedotin.

The preliminary safety and efficacy of SONALA-001 is being assessed as part of the SDT-201 study in diffuse intrinsic pontine glioma.
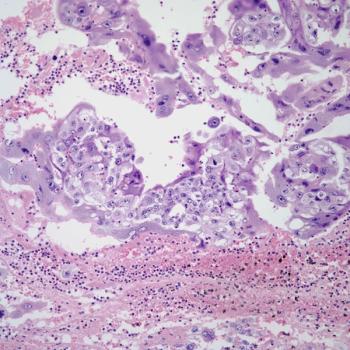
Investigators report that loss of MMR mechanism is not predictive of response to chemotherapy or pembrolizumab in patients with stage III or IVA, stage IVB, or recurrent endometrial cancer.

The safety profile of durvalumab plus TACE and bevacizumab in patients with hepatocellular carcinoma in the phase 3 EMERALD-1 study appears to consistent with previous findings.

Adagrasib, which now has a positive CHMP opinion, was previously examined as part of the phase 1/2 KRYSTAL-1 study.

If momelotinib receives approval in the European Union, it will become the only agent for the treatment of myelofibrosis coupled with moderate to severe anemia.
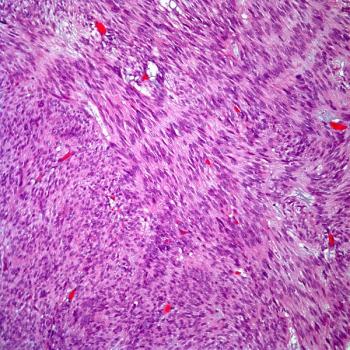
CHM 2101 will be assessed as part of a phase 1A/B clinical study in a population of patients diagnosed with advanced colorectal cancer, gastric cancer, and neuroendocrine cancer.
AL102 is being assessed as part of the phase 3 RINGSIDE trial in patients with desmoid tumors.

CMG901 demonstrated a well-tolerated safety profile in patients with CLDN18.2-positive, advanced gastric/gastroesophageal junction cancer.

CB-012 is set to be investigated as part of the phase 1 AMpLify trial in patients with relapsed/refractory acute myeloid leukemia.

The Canadian approval of relugolix for advanced prostate cancer is based on findings from the phase 3 HERO study.

A phase 1 study will evaluate TERN-701’s safety, efficacy, and pharmacokinetics in a population diagnosed with chronic myeloid leukemia.

Investigators report that bendamustine/rituximab debulking appears to decrease tumor lysis syndrome risk in patients with previously untreated chronic lymphocytic leukemia.

Patients diagnosed with BRAF V600E–mutant, metastatic non–small cell lung cancer are now able to undergo treatment with combination encorafenib and binimetinib.

LSAM-PTX plus standard of care appears efficacious in a small population of patients with locally advanced pancreatic cancer.

Over half of all patients with small cell neuroendocrine prostate cancer treated with BXCL701 and pembrolizumab were alive at 1 year in a phase 2 trial.

In this September edition of Snap Recap, we share our highlights from Prostate Cancer Awareness Month, news in the breast cancer space, and the latest FDA updates.

Diagnostic CT-enabled radiation therapy also reduces patient-reported time burden in the palliative setting.

Two phase 2 studies are assessing the efficacy of BDC-1001 in several disease states, including colorectal, gastroesophageal, endometrial, and breast cancer.

The marketing authorization application for imetelstat in lower-risk myelodysplastic syndrome is based on findings from the phase 3 IMerge trial.

Alisertib will be assessed in patients with small cell lung cancer as part of the phase 2 Study PUMA-ALI-4201.