
The biologic license application for odronextamab in relapsed/refractory follicular lymphoma or diffuse large B-cell lymphoma is based on data from the phase 1 ELM- 1 and phase 2 ELM-2 studies.

Your AI-Trained Oncology Knowledge Connection!


Hayley Virgil is a senior editor with CancerNetwork. When she isn't traveling to conferences and championing health equity in the oncology space, she can be found hiking, foraging wild plants, gardening, sewing ballgowns, practicing embroidery, or playing video games.

The biologic license application for odronextamab in relapsed/refractory follicular lymphoma or diffuse large B-cell lymphoma is based on data from the phase 1 ELM- 1 and phase 2 ELM-2 studies.

Investigators report a low rate of high-grade hematologic adverse effects following treatment with induction Dara-KRd and consolidation double transplant in patients with high-risk, newly diagnosed multiple myeloma.

IDE161 is now eligible for inclusion in a development program allowing for expediated regulatory review following its FDA fast track designation status for advanced BRCA1/2-mutant ovarian cancer.

The safety and tolerability of IDE161 is being assessed as part of a phase 1 trial in solid malignancies including breast cancer.

MYTX-011 is being assessed as part of the phase 1 KisMET-01 study in patients with locally advanced, recurrent or metastatic non–small cell lung cancer.

Based on findings from the phase 1/2 BCHILD trial, the FDA approved bosutinib for pediatric chronic myelogenous leukemia.

The conditional marketing approval of epcoritamab in relapsed/refractory diffuse large B-cell lymphoma is based on data from the phase 1/2 EPCORE NHL-1 study.

Investigators of the phase 3 CheckMate-77T trial report positive findings for patients with nonmetastatic non–small cell lung cancer who were treated with neoadjuvant nivolumab and chemotherapy.

Monique Gary, DO, MSc, FACS, discusses emerging data on early-onset cancers, including breast cancer which had the highest incidence in this population.

The agent, which is a recombinant Erwinia asparaginase or crisantaspase, can be given to those with acute lymphoblastic leukemia and lymphoblastic lymphoma intravenously and intramuscularly.

The regulatory decision to approve elacestrant in European patients with estrogen receptor–positive, HER2-negative locally advanced or metastatic breast cancer and an activating ESR1 mutation was based on data from the phase 3 EMERALD study.
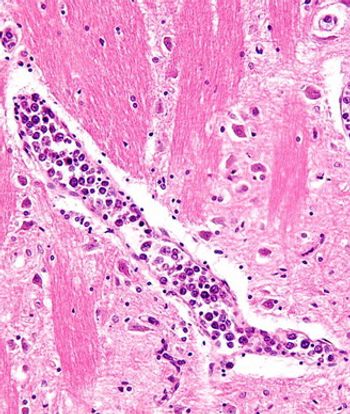
Several discrepancies in care must be considered to properly address psychological needs in patients with lymphoma, including patient/doctor communication, information provision, and applying distress guidelines.

The August CancerNetwork Snap Recap takes a look back at key FDA news updates, as well as expert perspectives on the chemotherapy shortage.

Across several clinical trials, Unfold AI appears to help in prostate cancer treatment selection and follow-up.

A single dose of motixafortide results in achievement of stem cell collection goal in patients with multiple myeloma.

A planned phase 1 trial will examine CDK12/13 inhibitor CT7439 in patients with several types of solid tumors, including breast and ovarian cancer, as well as Ewing’s sarcoma.

Marisol Miranda-Galvis, DDS, MS, PhD, highlights the importance of identifying populations of patients with hematologic cancer who are underserved and using socially targeted solutions.

Second-line end points of the TRICOTEL trial assessing atezolizumab plus vemurafenib and cobimetinib in melanoma with central nervous system metastases appear consistent with primary efficacy end points.

The FDA okayed 2 breakthrough therapy designations for fam-trastuzumab deruxtecan-nxki in HER2-positive solid tumors and colorectal cancer, which are based on data from the phase 2 DESTINY-PanTumor02 and DESTINY-CRC02 trials.

Despite seeming to elicit more pathological complete responses in patients with hormone receptor–positive, HER2-negative triple-negative breast cancer receiving neoadjuvant chemotherapy, diet and exercise did not affect relative dose intensity.

Investigators assess edoxaban as part of a multicenter, open-label superiority trial in patients with active cancer and newly diagnosed isolate distal deep vein thrombosis.
Findings from the LUMINA study indicate that it may be feasible to omit radiotherapy in patients 55 years and over with T1N0, grade 1/2 luminal A breast cancer.

Investigators report that Tumor Treating Fields and standard of care therapy should be considered as a treatment for non–small cell lung cancer in the metastatic setting.

Data from the phase 3 KEYNOTE-811 trial highlight a trend towards improved overall survival among patients with HER2-positive, PD-L1–positive advanced gastric or gastroesophageal junction adenocarcinoma receiving pembrolizumab, trastuzumab, and chemotherapy.
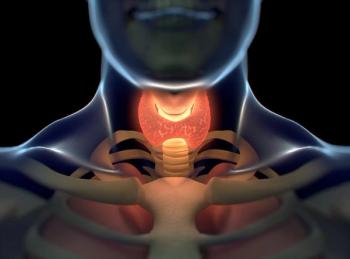
Positive data from a prespecified interim efficacy analysis of the phase 3 LIBRETTO-531 trial support selpercatinib as a potential treatment in advanced, RET-mutant medullary thyroid cancer.
Data from a next-generation sequencing analysis indicate that XPO1 may be a novel biomarker predictive of a decreased time to first treatment in patients with early-stage chronic lymphocytic leukemia.

The first and only dual action tablet, niraparib and abiraterone acetate, is now available for use in patients with BRCA-positive, metastatic castration-resistant prostate cancer following its approval by the FDA.

Continuing multidisciplinary discussions during the chemotherapy shortage is important for delivering the best possible care for each patient with breast cancer, according to Maryam Lustberg, MD, MPH.

Using the 31-gene expression profile assay may help lead to more personalized treatment strategies in patients with cutaneous melanoma, according to Aaron Farberg, MD.

Flotufolastat F 18 is indicated for use in imaging PSMA-positive prostate cancer lesions in patients who are likely to have metastatic disease and may be able to undergo initial definitive therapy, as well as those who may have recurrent disease.