
Investigators highlight the importance of building partnerships between CAR T-cell centers and external hospitals to increase treatment referrals and access to clinical trials for patients with B-cell acute lymphoblastic leukemia.

Your AI-Trained Oncology Knowledge Connection!


Hayley Virgil is a senior editor with CancerNetwork. When she isn't traveling to conferences and championing health equity in the oncology space, she can be found hiking, foraging wild plants, gardening, sewing ballgowns, practicing embroidery, or playing video games.

Investigators highlight the importance of building partnerships between CAR T-cell centers and external hospitals to increase treatment referrals and access to clinical trials for patients with B-cell acute lymphoblastic leukemia.

Incorporating nursing-led palliative care may help to improve advanced care planning among those with advanced cancer, especially those without access to specialty palliative care.
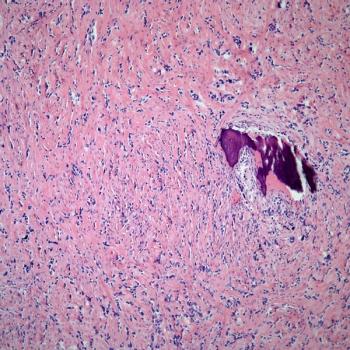
TP-1287 is under investigation as part of a phase 1 study in patients with metastatic or progressive solid tumors, including Ewing sarcoma, that are refractory or intolerant to other established therapies.
Patients with advanced high-grade epithelial BRCA mutation–negative ovarian cancer appear to benefit from treatment with olaparib and durvalumab plus bevacizumab and chemotherapy.

A first-in-human phase 1/2a trial will assess anti-PAUF monoclonal antibody PBP1510 in advanced/metastatic pancreatic cancer.

In addition to granting fast track designation to RRx-001, the FDA accepts an investigational new drug application for the agent to reduce and/or prevent radiation- and chemotherapy-associated oral mucositis.

The new drug application that was submitted to the FDA for fruquintinib in refractory, metastatic colorectal cancer is supported by data from the phase 3 FRESCO-2 trial.

Data from the phase 3 TIGeR-PaC trial indicates that patients with locally advanced pancreatic cancer experienced notable survival benefit with RenovoGem vs chemotherapy.

Patients with advanced endometrial cancer experience early responses to lenvatinib plus pembrolizumab in both patients who were mismatch repair proficient and all-comers.

A post hoc analysis demonstrated that dose modifications to frontline niraparib maintenance therapy did not affect progression-free survival among patients with newly diagnosed, advanced ovarian cancer.

Vadim Gushchin, MD, says that treatment with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy should not be limited by concerns of negatively impacting health-related quality of life in ovarian cancer.

Patients with metastatic or recurrent locally advanced Merkel cell carcinoma can now receive treatment with retifanlimab-dlwr following its accelerated approval by the FDA.
Kidney Cancer Association president Gretchen Vaughan discusses improvements being rolled out by the organization and the importance of multidisciplinary care.

National Comprehensive Cancer Network guidelines will now include ripretinib as the ideal second-line treatment for patients with unresectable/metastatic gastrointestinal stromal tumors who are intolerant to sunitinib.

Investigators indicate that strategies are necessary to identify patients with extensive-stage small cell lung cancer who won’t benefit from immunotherapy-based treatment.

The final analysis of the phase 2/3 CCTG IND.227/KEYNOTE-483 trial identifies a statistically significant survival benefit with pembrolizumab and chemotherapy vs chemotherapy alone in the first-line setting for patients with unresectable advanced or metastatic malignant pleural mesothelioma.

Patients with hormone receptor–positive, HER2-negative, node-positive early breast cancer at a high risk of relapse who are eligible for treatment with abemaciclib can be identified via nodal status as well as tumor size and grade.

Patients with BRCA-positive metastatic castration-resistant prostate cancer may benefit from treatment with niraparib plus dual action abiraterone acetate tablets and prednisone, a new drug application for which was submitted to the FDA.
Extended follow-up findings further support the use of nivolumab as a standard of care in high-risk muscle-invasive urothelial carcinoma following radical resection.

A medical oncologist and research audiologist from St. Jude Children’s Research Hospital discuss how sodium thiosulfate injection may improve quality of life by preventing cisplatin-associated ototoxicities in pediatric patients with localized non-metastatic tumors, although more research is needed.
In a meeting with the FDA’s Oncologic Drugs Advisory Committee experts discussed findings that have read out with regard to dostarlimab for patients with mismatch repair deficiency/microsatellite instability–high locally advanced rectal cancer.

Patients with mismatch repair deficient recurrent/advanced endometrial cancer can receive treatment with dostarlimab-gxly following its full approval by the FDA.

“Being a patient advocate means not so much putting ourselves in the patient's shoes but asking the right questions to find out what those barriers to care are,” says an oncology survivorship navigator from Sibley Memorial Hospital, Johns Hopkins Medicine.

Patients with tenosynovial giant cell tumors, a rare locally aggressive neoplasm, who are unable to undergo surgery may benefit from treatment with pimicotinib.

The FDA has approved Guardant 360 CDx as a companion diagnostic for elacestrant in patients with ESR1-mutant, estrogen receptor–positive, HER2-negative advanced or metastatic breast cancer.
Tamibarotene is a first-in-class oral first-in-class selective retinoic acid receptor α agonist that is being evaluated in combination with azacitidine for newly diagnosed higher-risk myelodysplastic syndrome.

An expert from the Smilow Cancer Hospital and Yale Cancer Center indicates that findings from the ctDNA analysis of the phase 3 INTRIGUE study were “provocative” and that the subsequent phase 3 INSIGHT study has the potential to be practice changing should data be positive.

Molecular responders by G360 response algorithm with advanced colorectal cancer appear to have significantly lengthened time on treatment and overall survival with all regimens vs patients who did not respond.

Patients with chronic lymphocytic leukemia and small lymphocytic leukemia can now receive treatment with zanubrutinib following its approval by the FDA.

The FDA gave the dual epigenetic modulator JBI-802 an orphan drug designation to treat small cell lung cancer and acute myeloid leukemia.