The FDA’s Oncologic Drugs Advisory Committee voted against continued use of melphalan flufenamide for the treatment of relapsed/refractory multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


Hayley Virgil is a senior editor with CancerNetwork. When she isn't traveling to conferences and championing health equity in the oncology space, she can be found hiking, foraging wild plants, gardening, sewing ballgowns, practicing embroidery, or playing video games.

The FDA’s Oncologic Drugs Advisory Committee voted against continued use of melphalan flufenamide for the treatment of relapsed/refractory multiple myeloma.

Patients with RET fusion–positive locally advanced/metastatic non–small cell lung cancer can now receive treatment with RET inhibitor selpercatinib, which was approved by the FDA.

Patients with RET fusion–positive locally advanced or metastatic solid tumors can now receive treatment with selpercatinib following its approval by the FDA.
Patients with pancreatic cancer may benefit from treatment with ATG-101, which was granted orphan drug designation by the FDA.

Sodium thiosulfate, which appears to be effective in decreasing the risk for hearing loss related to treatment with cisplatin, received FDA approval in pediatric patients with localized, non-metastatic solid tumors.
Patients with previously treated, metastatic, HER2-positive colorectal cancer may benefit from treatment with tucatinib and trastuzumab, which was granted priority review by the FDA.

Patients with newly diagnosed ovarian cancer with or without a high risk of progression experienced a progression-free survival benefit from treatment with maintenance rucaparib compared with placebo regardless of molecular characteristics.
Despite the combination of enzalutamide plus abiraterone acetate and prednisolone (AAP) falling short in patients with metastatic hormone-sensitive prostate cancer, although androgen deprivation therapy plus AAP resulted in a clinically meaningful overall survival benefit.

Guidelines from the American Society for Radiation Oncology on the use of radiation therapy for patients with IDH-mutant glioma included strong recommendations for close surveillance in IDH-mutant, 1p/19q codeleted, WHO grade 2 oligodendroglioma with no high-risk features and adjuvant radiotherapy for those with WHO grade 3 disease.
Ranjit Nair, MD, discusses the promise of sugemalimab, both as a single-agent and as part of a combination regimen, in patients with relapsed/refractory extranodal natural killer/T-cell lymphoma.

Results from the phase 2 ExIST trial indicated that the addition of galunisertib to neoadjuvant chemoradiotherapy for patients with locally advanced rectal cancer resulted in an improvement in responses.

Patients with locally advanced or metastatic biliary tract cancer can now receive treatment with durvalumab plus gemcitabine following the combination’s approval by the FDA.

Findings from a study revealed a notable reduction in the risk of prostate cancer–specific mortality in Black United States veterans who underwent prostate-specific antigen screening, highlighting the potential importance of undergoing annual screenings in this patient population.
Blood circulating soluble proteins may be an effective and accessible biomarker for predicting response to immunotherapy among patients with squamous cell lung cancer.

Patients with previously treated KRAS G12C–mutated non–small cell lung cancer experienced superior survival benefit following treatment with sotorasib compared with docetaxel.

A promising 5-year locoregional recurrence rate highlighted that it is likely safety to de-escalate treatment with locoregional radiotherapy in a population of patients with cT1 to cT2, node-positive breast cancer that has been treated using primary chemotherapy and surgery.

The FDA has placed a full clinical hold on a phase 1 trial assessing FHD-286 as a potential treatment for patients with relapsed/refractory acute myelogenous leukemia and myelodysplastic syndrome due to concerns around suspected cases of fatal differentiation syndrome that may be associated with the agent.

Pemigatinib had been approved by the FDA as a treatment for patients with FGFR1 rearranged relapsed/refractory myeloid/lymphoid neoplasms.

The European Commission has announced the first global approval of teclistamab for previously treated relapsed/refractory multiple myeloma following its conditional marketing authorization.

In a population of patients with recurrent mismatch repair proficient endometrial cancer, treatment with avelumab and talazoparib appeared to be well tolerated.

Adult patients with acute myeloid leukemia who are not eligible for treatment with standard induction chemotherapy may benefit from treatment with decitabine and cedazuridine, the marketing authorization application for which was accepted by the European Medicines Agency.

Early findings indicate that CLN-081 may hold promise in patients with advanced EGFR exon 20 insertion mutation–positive non–small cell lung cancer.
Patients with clear cell renal cell carcinoma may experience successfully downstaging of venous tumor thrombus extent with neoadjuvant axitinib.
A long-term data readout from the phase 3 IT-MATTERS trial highlighted the potential of leukocyte interleukin injection as a treatment for patients with locally advanced primary squamous cell carcinoma of the head and neck, oral cavity, and soft palate.
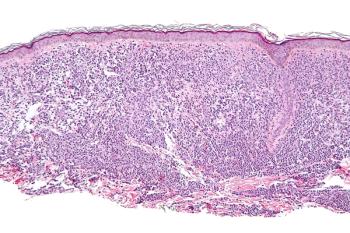
Primary and secondary end points from the phase 2 PIONEER trial assessing avapritinib in nonadvanced systemic mastocytosis were met.

In an update to the phase 3 CANOPY-A trial, investigators reported that canakinumab did not significantly improve disease-free survival over placebo in patients with completely resected non–small cell lung cancer.
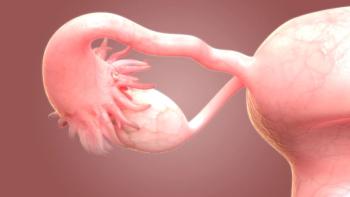
In a population of patients with cervical intraepithelial neoplasia and stage IA1 cervical cancer, certain local treatments, such as radical excision and ablation, were associated with treatment outcomes and risk of preterm birth.

A partial clinical hold placed on the phase 1/2 TakeAim Lymphoma trial was lifted after a strategy to identify and manage rhabdomyolysis was agreed upon.
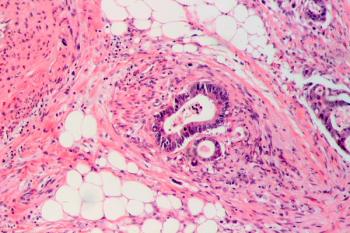
Investigators found that patients with non–gain-of-function TP53-mutant right-sided metastatic colorectal cancer and gain-of-function TP53-mutant left-sided tumors had poorer survival vs their counterparts.
Patients with previously untreated diffuse large B-cell lymphoma may benefit from treatment with polatuzumab vedotin-piiq plus rituximab, cyclophosphamide, doxorubicin, and prednisone, for which a supplemental biologics license application was accepted by the FDA.