
The FDA has approved a new device that allows for the expansion of soft tissue in two-stage breast reconstruction surgery conducted after mastectomy.

Your AI-Trained Oncology Knowledge Connection!


The FDA has approved a new device that allows for the expansion of soft tissue in two-stage breast reconstruction surgery conducted after mastectomy.

Although the use of sorafenib in patients with treatment-naive metastatic renal cell carcinoma demonstrated clinical benefit, a phase IIb study did not support the use of dose escalation in these patients.
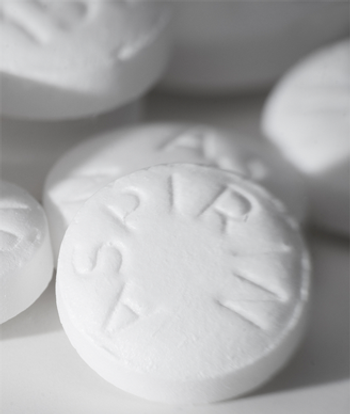
The regular use of aspirin reduced the risk for pancreatic cancer by almost 50%, according to the results of a Chinese study.

A study found that about 20% of women with breast cancer did not complete their prescribed endocrine therapy.

The use of anti-CD19 chimeric antigen receptor T cells induced a nearly sixfold higher rate of complete response compared with historical outcomes in patients with refractory, aggressive non-Hodgkin lymphoma.

Reducing the use of indoor tanning by enforcing an age restriction could potentially reduce melanoma incidence, mortality, and the costs associated with treating the disease, according to results of an economic analysis.

Postmenopausal women with breast cancer who took aromatase inhibitors (AI) had endothelial dysfunction, according to the results of a study.

With the help of a scalp-cooling device, 50% of women with breast cancer undergoing neoadjuvant or adjuvant chemotherapy were able to prevent hair loss, according to the results of the SCALP trial.

Treatment with buparlisib in combination with endocrine therapy significantly improved PFS compared with placebo in patients with HR-positive advanced breast cancer that had progressed after treatment with everolimus plus exemestane.

The addition of estrogen deprivation to neoadjuvant chemotherapy did not significantly affect pathologic complete response in women with HR-positive, HER2-positive breast cancer.

Pembrolizumab was associated with clinically meaningful improvements in health-related quality of life compared with platinum-based chemotherapy in NSCLC patients.

The PD-L1 inhibitor atezolizumab significantly improved overall survival compared with docetaxel across subgroups of patients with non–small-cell lung cancer.

Second-line treatment with osimertinib resulted in significantly better progression-free survival compared with chemotherapy alone in patients with EGFR-T790M positive NSCLC, according to the results of the AURA3 trial.

Patients treated with first-line ceritinib had a 45% reduction in the risk for progression of advanced ALK-positive NSCLC compared with chemotherapy.

T-cell therapy targeting CD22, a protein found on the surface of leukemic cells, was safe and feasible in a small and ongoing study of patients with ALL.

Many patients with stable CML may be able to safely decrease their dose of tyrosine kinase inhibitor to half of the standard dose and improve TKI-related side effects, according to the results of the DESTINY trial.

Cessation of tyrosine kinase inhibitor therapy may be possible in chronic myeloid leukemia patients with deep molecular response, according to the results of the Euro-Ski trial.

Administration of bb2121, a novel anti–B-cell maturation antigen CAR T-cell therapy, produced anti-tumor responses in heavily pretreated patients with relapsed/refractory multiple myeloma, according to interim data from a phase I trial.

Combining standard chemotherapy with vadastuximab talirine was safe and well-tolerated in newly diagnosed acute myeloid leukemia.

The combination of CB-839, a selective inhibitor of glutaminase, and everolimus seems to have disease activity in patients with advanced renal cell carcinoma.

The FDA has approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone for patients with multiple myeloma who have received at least one prior therapy.

Researchers have identified characteristics associated with improved outcomes when treating BRAF-mutated advanced melanoma with the combination of dabrafenib and trametinib.

Preoperative testing of thyroid nodules for RAS mutations showed that nodules that tested positive for HRAS and NRAS were associated with a substantial risk for cancer.

By 2025 there will be an increasing number of people living with CLL due to improved survival conferred by emerging targeted therapies; however, the annual cost of CLL management for both patients and providers may impose a significant financial burden.

Patients with metastatic renal cell carcinoma treated with sunitinib often experience treatment-related hypertension and require rigorous blood pressure control.

Patients with AML or MDS with unfavorable risk cytogenetic profiles, TP53 mutations, or both had good clinical response and robust mutation clearance with decitabine.

The use of radiotherapy may have an important role in optimizing first-line treatment for patients with early-stage extranodal natural killer/T-cell lymphoma.

The use of inpatient palliative care services among adult patients undergoing hematopoietic stem cell transplantation for a hematologic malignancy results in smaller decreases in quality-of-life outcomes compared with standard transplant care.

Patients with ER-positive, HER2-negative advanced breast cancer had significant delays in progression when palbociclib was added to letrozole treatment, according to the double-blind PALOMA-2 study.

Patients with multiple myeloma may have improved tolerance of panobinostat when combined with low-dose thalidomide and subcutaneous bortezomib, according to the results of the phase I/II MUK-six trial.