
An FDA filing decision is anticipated before the end of 2024 for avutometinib/defactinib in recurrent KRAS-mutant low-grade serous ovarian cancer.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

An FDA filing decision is anticipated before the end of 2024 for avutometinib/defactinib in recurrent KRAS-mutant low-grade serous ovarian cancer.

Findings speak to the need of cultural, racial, and ethnic inclusion when designing breast cancer trials and developing patient-reported outcome measures.

Investigators will present topline data from the phase 2/3 study of uproleselan/chemotherapy at a future medical meeting.

Data from the AcceleRET-Lung trial show an imbalanced risk of severe and fatal infection with pralsetinib for patients with RET fusion-positive NSCLC.

Data from the ASC4FIRST trial support the accelerated approval of asciminib in this CML population.

The expanded approval of methotrexate may offer a convenient alternative to pediatric patients who have difficulty swallowing pills.

Data from TROPiCS-04 did not meet the primary end point of overall survival among those with locally advanced or metastatic urothelial cancer.

Phase 1 expansion data may support potential combination development for IDE397 in NSCLC and urothelial cancer harboring MTAP deletions.

Promising phase 1 data appear to support further evaluation of ZL-1310 as a treatment for patients with extensive-stage small cell lung cancer.

The FDA has set a Prescription Drug User Fee Act date of April 26, 2025, for TLX101-CDx as an imaging agent for patients with glioma.

Investigators from the United States, United Kingdom, Canada, and Germany look to conduct a primary prevention trial in patients with Li-Fraumeni syndrome.

Data from KEYNOTE-868 and KEYNOTE-A18 support the approval of pembrolizumab-based therapy in endometrial and cervical cancer, respectively.

The agency places a partial clinical hold on the phase 3 PRESERVE-003 trial due to varying results between the squamous and nonsquamous NSCLC cohorts.

Investigators are assessing the feasibility, safety, and efficacy of MTX110 for patients with recurrent glioblastoma as part of the phase 1 MAGIC-1 study.

Offering certain radiotherapy modalities based on disease burden may play a role in the outcomes of those with ES-SCLC, according to James Ninia, MD.

Updated findings from RAMP 201 show that avutometinib/defactinib is generally well tolerated among those with low-grade serous ovarian cancer.

Phase 3 data show that nivolumab/AVD may also reduce long-term toxicities vs brentuximab vedotin/AVD in advanced Hodgkin lymphoma.

Bin Gui, MD, discussed how ultra-hypofractionated radiotherapy may be a convenient treatment option for elderly patients with early breast cancer.

Data from the phase 2 SAVANNAH trial may support savolitinib as a new treatment option following standard-of-care osimertinib in EGFR-mutated NSCLC.

Support for the approval comes from phase 3 LUNAR study findings indicating TTFields significantly prolonged OS compared with standard of care alone.
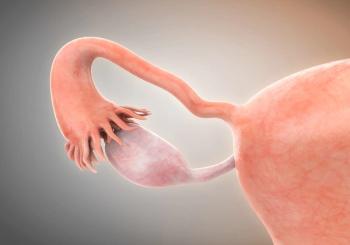
Subgroup data indicate a positive efficacy trend for TG4001 plus avelumab among patients with cervical cancer.

Omitting bolus 5-fluouracil in chemotherapy-based treatment for patients with gastrointestinal cancers may reduce adverse effects and healthcare costs.
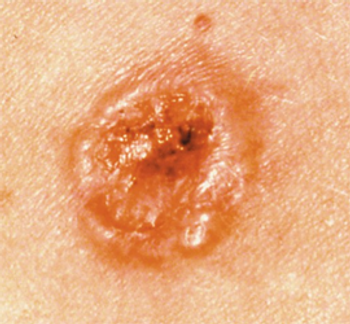
Phase 1b findings may affirm the therapeutic potential of IMA203 for patients with previously treated metastatic melanoma.
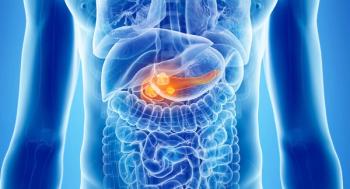
Results from the CANFOUR trial of combining nadunolimab with chemotherapy appear to prolong overall survival among those with advanced or metastatic PDAC.

Patients with non–small cell lung cancer and larger brain metastases may benefit most from the addition of up-front stereotactic radiosurgery to TKIs.

Data from the INAVO120 trial support the approval of the inavolisib combination for those with PIK3CA-mutant breast cancer.

Detailed results from the phase 3 TALAPRO-2 trial will be submitted for presentation at a future medical meeting and shared with global health authorities.

Phase 2 data indicate that the use of DetermaIO was predictive of a pathologic complete response with the addition of immunotherapy to chemotherapy in TNBC.

Data also show an improvement in major pathological response in the perioperative pembrolizumab arm of the KEYNOTE-689 trial.

Data from the LUMINOSITY trial support the application for telisotuzumab vedotin in nonsquamous NSCLC harboring c-Met protein overexpression.