
Phase 2 data support further evaluation of nivolumab plus standard radiotherapy in patients with Gleason grade 5 prostate cancer.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Phase 2 data support further evaluation of nivolumab plus standard radiotherapy in patients with Gleason grade 5 prostate cancer.
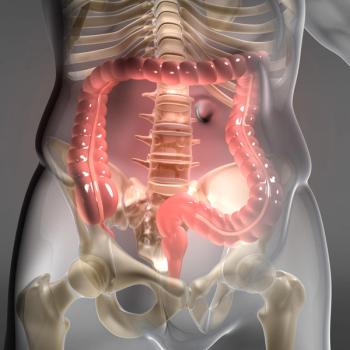
Data from the BLUE-C trial support the approval of the Cologuard Plus test for colorectal cancer screening among at-risk individuals.

Data show that twice-daily radiotherapy may confer improved survival vs once-daily radiation in patients with limited-stage small cell lung cancer.

The FDA has set a Prescription Drug User Fee Act date in the first quarter of 2025 for the potential approval of acalabrutinib in previously untreated MCL.

Use of DCISionRT may open new options for tailored treatments among patients with HER2-positive ductal carcinoma in situ.

Investigators anticipate launching the dose-expansion portion of the phase 1 HDP-101-01 trial in 2025.

UNITED is the first trial to assess MR-guided weekly adaptive on-line and real-time radiotherapy with clinical target volume margin reductions.

Data from DESTINY-Breast06 support the priority review designation for T-DXd as a treatment for HER2-low or HER2-ultralow breast cancer.
Data show no differences in bowel function between treatment arms based on factors including age, disease risk, and fractionation schedule.
Combining talquetamab with teclistamab elicits responses even among those with extramedullary disease in the RedirecTT-1 trial.

Eleven votes were cast against the favorability of using anti–PD-1 inhibitors in patients with ESCC and a PD-L1 expression of less than 1.

Ten votes were cast against PD-L1 as an efficacy biomarker for therapies intended to treat patients with gastric or GEJ adenocarcinoma.

Responses appear to improve over time in those with transplant-eligible newly diagnosed multiple who receive the belantamab mafodotin-based combination.
Updated findings from the CARES-310 trial support the resubmitted application for camrelizumab/rivoceranib in unresectable hepatocellular carcinoma.

Data from the GLOW and SPOTLIGHT trials support the European Commission’s approval of zolbetuximab for patients with CLDN18.2-positive gastric cancer.

Pelareorep plus paclitaxel improved the overall response rate vs paclitaxel monotherapy among patients in the phase 2 BRACELET-1 study.

ESMO 2024 saw a wide range of potentially practice-changing data across multiple oncology disciplines such as the breast cancer and lung cancer fields.

Data from the KEYNOTE-483 trial support the FDA approval of the pembrolizumab-based combination in this pleural mesothelioma population.

HERTHENA-Lung02 investigators will present further data from the trial at a future medical meeting.
Data from NATALEE continue to support the addition of ribociclib to adjuvant nonsteroidal aromatase inhibitors in HR-positive, HER2-negative breast cancer.

The magnitude of benefit with durvalumab was particularly consistent within prophylactic cranial irradiation and radiation subgroups in the ADRIATIC trial.
Early data appear to highlight encouraging efficacy and tolerability outcomes with Bria-IMT in patients with metastatic breast cancer.
Developers anticipate releasing full efficacy results from the phase 2 THIO-101 trial in late 2024.

HER2DX demonstrated the ability to select patients who are more likely to achieve disease control with anti-HER2 standard of care in the CLEOPATRA trial.

Treatment with RLY-2608 plus fulvestrant appears well tolerated among patients with PI3Kα-mutated breast cancer in the ReDiscover trial.

Phase 1b data also show encouraging preliminary intracranial activity with zongertinib among patients with HER2-mutant non–small cell lung cancer.

Earlier use of liso-cel may improve responses in patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma.

Phase 1 data may warrant further investigation of TLX101 plus external beam radiation therapy for patients with recurrent glioblastoma.
Data from LITESPARK-005 establish belzutifan as a treatment option in advanced RCC following prior immune checkpoint and antiangiogenic therapies.

Treatment with CB-012 for patients with relapsed/refractory acute myeloid leukemia is under evaluation as part of the phase 1 AMpLify trial.