Phase 1b/2 data show antitumor activity with zanidatamab/evorpacept, including among heavily pretreated patients with HER2-low metastatic breast cancer.

Your AI-Trained Oncology Knowledge Connection!


Russ Conroy is an Associate Editor for CancerNetwork. He grew up in Hillsborough, New Jersey, and graduated from Rutgers University-New Brunswick in 2022.
On the weekends, he likes to unwind by playing video games with friends, tailgating at Rutgers football games with his family, or building his music collection with a visit to Princeton Record Exchange.

Phase 1b/2 data show antitumor activity with zanidatamab/evorpacept, including among heavily pretreated patients with HER2-low metastatic breast cancer.

Phase 2 data indicate that CD8 status may serve as a biomarker for predicting treatment efficacy with tislelizumab-based treatment in TNBC.

Data from the phase 3 OPERA trial support contact X-ray brachytherapy boost as a treatment option for preserving organs in smaller rectal adenocarcinoma tumors.

Data from the phase 3 MSB-GVHD001 trial support the FDA approval of remestemcel-L in pediatric SR-aGVHD.

SABCS 2024 saw a variety of potentially practice-changing findings on novel antibody drug conjugates, biomarker data, and surgical interventions.
Meta-analysis data show moderate decreases in distant recurrence and breast cancer mortality with immediate surgery after longer follow-up.
An artificial intelligence model may help move patients into treatment pathways for pancreatic cancer quicker, says Russell C. Langan, MD, FACS, FSSO.

Phase 2 data support epcoritamab monotherapy as a promising chemotherapy-free option for older patients with newly diagnosed LBCL.

A panel of cancer survivorship experts outline major areas of focus and care models for improving outcomes among pediatric and adult survivors of cancer.

The IPSS-del(5q) Scoring System shows that factors including male sex, cytopenias, and complex genetic background worsen the prognosis of MDS-del(5q).
The launch of this pazopanib tablet formulation for patients with RCC or sarcoma is anticipated in the fourth quarter of 2025.
Shubham Pant, MD, MBBS, highlights how pan-RAS inhibitors, RAS-directed vaccines, and biomarker testing can improve outcomes in pancreatic cancer.

Data show an early trend towards improved overall survival with capivasertib plus abiraterone and androgen deprivation therapy in CAPItello-281.

The updated labeling also includes new information on the recommended dosage of fludarabine phosphate when given with cyclophosphamide and rituximab.

Data from the phase 2/3 KEYNOTE-483 trial support the CHMP’s recommendation for approving pembrolizumab/chemotherapy in the European Union.
Data from the phase 2b ReNeu trial may support mirdametinib as a valuable new treatment option for adults and children with NF1-PN.

Survival data from the interim analysis of the phase 3 DREAMM-7 trial will be presented at the 2024 ASH Annual Meeting.
Treatment with pimicotinib also yields meaningful improvements in stiffness and pain symptoms among patients with TGCT in the phase 3 MANEUVER study.

Data from TROPION-Lung05, TROPION-Lung01, and TROPION-PanTumor01 support the new BLA for dato-DXd in advanced or metastatic EGFR-mutated NSCLC.

Glycan editing of cell surface glycans with E-602 may represent a novel therapeutic approach among patients with cancer.

Phase 1 data highlight a manageable safety profile with IMA203 among patients with melanoma and other PRAME-positive solid tumors.

Data from the phase 3 AQUILA study support the applications for subcutaneous daratumumab in high-risk smoldering multiple myeloma.

The 18-month overall survival rate with the mitazalimab combination in OPTIMIZE-1 exceeds a historical rate achieved with FOLFIRINOX alone.

Phase 2 data support a favorable risk/benefit profile with valemetostat in patients with relapsed/refractory peripheral T-cell lymphoma.

Treatment with domvanalimab/zimberelimab also improved progression-free survival vs platinum-based chemotherapy in the phase 3 ARC-10 study.

The updated Prescription Drug User Fee Act date for zenocutuzumab in these indications is February 4, 2025.
Treatment with (Z)-endoxifen yielded no changes in hematological safety tests among patients enrolled on the phase 2 KARISMA-Endoxifen study.

Consolidative hematopoietic cell transplantation also confers improved progression-free survival among those with relapsed/refractory B-ALL.
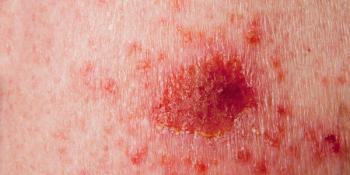
Phase 3 data may support the photodynamic therapy as a noninvasive treatment option for patients with superficial basal cell carcinoma.

Study data show that no patient with a response required opioids after a 24-hour post-procedure follow-up.