Colorectal Cancer
Latest News
Latest Videos

CME Content
More News
Tanios Bekaii-Saab, MD, provides a brief overview of the typical presentation and diagnosis of metastatic colorectal cancer.

Lead author Ibrahim Halil Sahin, MD, spoke with CancerNetwork® about his work investigating postoperative circulating tumor DNA dynamics in early-stage colon cancer as part of the CIRCULATE-US trial published in the journal ONCOLOGY®.
For patients with colon cancer, 3-month treatment with mFOLFOX6 or CAPOX improved safety outcomes without affecting efficacy vs 6-month treatment, according to data from the phase 3 ACHIEVE trial.
Opioid use disorder and overdose are important risks to consider in older patients diagnosed with stage II/III colorectal cancer, especially those who were opioid naïve.
Ardaman Shergill, MD, and Aparna Raj Parikh, MD, explore ongoing clinical investigations evaluating the use of circulating tumor DNA in the treatment of early-stage colon cancer.
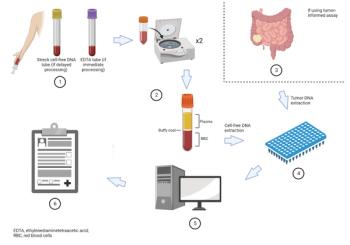
Ben Fangman, MD, and colleagues provide an overview of the use of circulating tumor DNA levels to detect minimal residual disease in colorectal cancer.
Data from the phase 3 SUNLIGHT study highlighted the increased survival benefit of trifluridine/tipiracil plus bevacizumab compared with trifluridine/tipiracil alone for the treatment of metastatic colorectal cancer.
Patients with metastatic colorectal cancer who were below a certain income level appear to be more susceptible to major financial hardship.
Chiara Cremolini, MD, PhD, discussed results from the phase 3 TRIPLETE study of triplet chemotherapy plus panitumumab in previously untreated patients with RAS/BRAF wild-type unresectable metastatic colorectal cancer.
FDA Grants Priority Review to Tucatinib Plus Trastuzumab for Previously Treated HER2+ Metastatic CRC
Patients with previously treated, metastatic, HER2-positive colorectal cancer may benefit from treatment with tucatinib and trastuzumab, which was granted priority review by the FDA.

Based on findings from a real-world retrospective analysis, Stacey A. Cohen, MD, discussed the prognostic value of post-surgical minimal residual disease detection in patients with stage I to III colorectal cancer.
Findings from the phase 1b/2 KRYSTAL-1 trial showed encouraging responses with adagrasib in patients with advanced KRAS G12C-mutant colorectal cancer.
Patients with mismatch repair deficient (dMMR) colon cancer experienced a high rate of major pathologic complete response following treatment with nivolumab and ipilimumab for 4 weeks.
Tucatinib as monotherapy and in combination with trastuzumab supported further investigation of the regimens in patients with metastatic HER2-positive colorectal cancer.

At the 2022 ASCO Annual Meeting, Christine Parseghian, MD, reviewed results from a phase 2 trial assessing efficacy of panitumumab plus or minus trametinib in patients with RAS/BRAF wild-type colorectal cancer and compared EGFR rechallenge strategies with available agents in the third-line setting.

Results from the phase 2 ExIST trial indicated that the addition of galunisertib to neoadjuvant chemoradiotherapy for patients with locally advanced rectal cancer resulted in an improvement in responses.
Three months of neoadjuvant chemotherapy may downstage early-stage rectal cancer and thereby reduce the need for total mesorectal excisions (TMEs), resulting in higher rates of organ preservation.

Christine Parseghian, MD, discussed the effects of rechallenging patients with a prior response to EGFR inhibition with panitumumab plus or minus the MEK inhibitor trametinib in RAS/BRAF wildtype, microsatellite stable colorectal cancer.

Cornelis J. A. Punt, MD, PhD, spoke about bevacizumab plus either FOLFOXIRI or FOLFOX/FOLFIRI in patients with unresectable colorectal liver metastases and right-sided or RAS/BRAF-mutant tumors and how these regimens may be used for curative-intent therapy.

Expert oncologists look toward future utilization of circulating tumor DNA testing and consider how the field of oncology may evolve.

Shared insight on how circulating tumor DNA may be used in real-world clinical practice to improve the value of cancer care.

Data from the phase 2 DYNAMIC trial show that a circulating tumor DNA–guided approach to adjuvant therapy selection may be feasible for patients with stage II colorectal cancer, but Tanios S. Bekaii-Saab, MD, is skeptical about its readiness for prime time.

Moving on to the second patient scenario, panelists elucidate the value of ctDNA in patients with triple-negative breast cancer.
Investigators found that patients with non–gain-of-function TP53-mutant right-sided metastatic colorectal cancer and gain-of-function TP53-mutant left-sided tumors had poorer survival vs their counterparts.

Data from the PARADIGM trial that were presented at 2022 ASCO demonstrated that panitumumab plus FOLFOX should be standard of care for patients with left-sided, RAS wild-type colorectal cancer, according to Tanios S. Bekaii-Saab, MD.