
Though physical activity guidelines are largely based on chronic diseases like cardiovascular disease and diabetes, these data suggest they are important to cancer prevention as well.

Your AI-Trained Oncology Knowledge Connection!


Though physical activity guidelines are largely based on chronic diseases like cardiovascular disease and diabetes, these data suggest they are important to cancer prevention as well.

The study evaluating itacitinib in combination with corticosteroids in patients with treatment-naïve acute graft-versus-host disease did not produce statistically significant results.

A phase I study showed brentuximab vedotin combined with a standard chemotherapy regimen of mitoxantrone, etoposide, and cytarabine may be safe and well tolerated in patients with relapsed/refractory acute myeloid leukemia.

Diego Villa, MD, FRCPC, elaborated on the progress made with bendamustine and rituximab as induction therapy for transplant eligible and ineligible patients with mantle cell lymphoma.
The expert hematologist from Plymouth University Medical Center discussed the long-term outcomes of patients receiving ibrutinib for mantle cell lymphoma at the ASH Annual Meeting and Exposition.

Bijal D. Shah, MD, discusses the progress made regarding CAR T-cell therapy and mantle cell lymphoma.
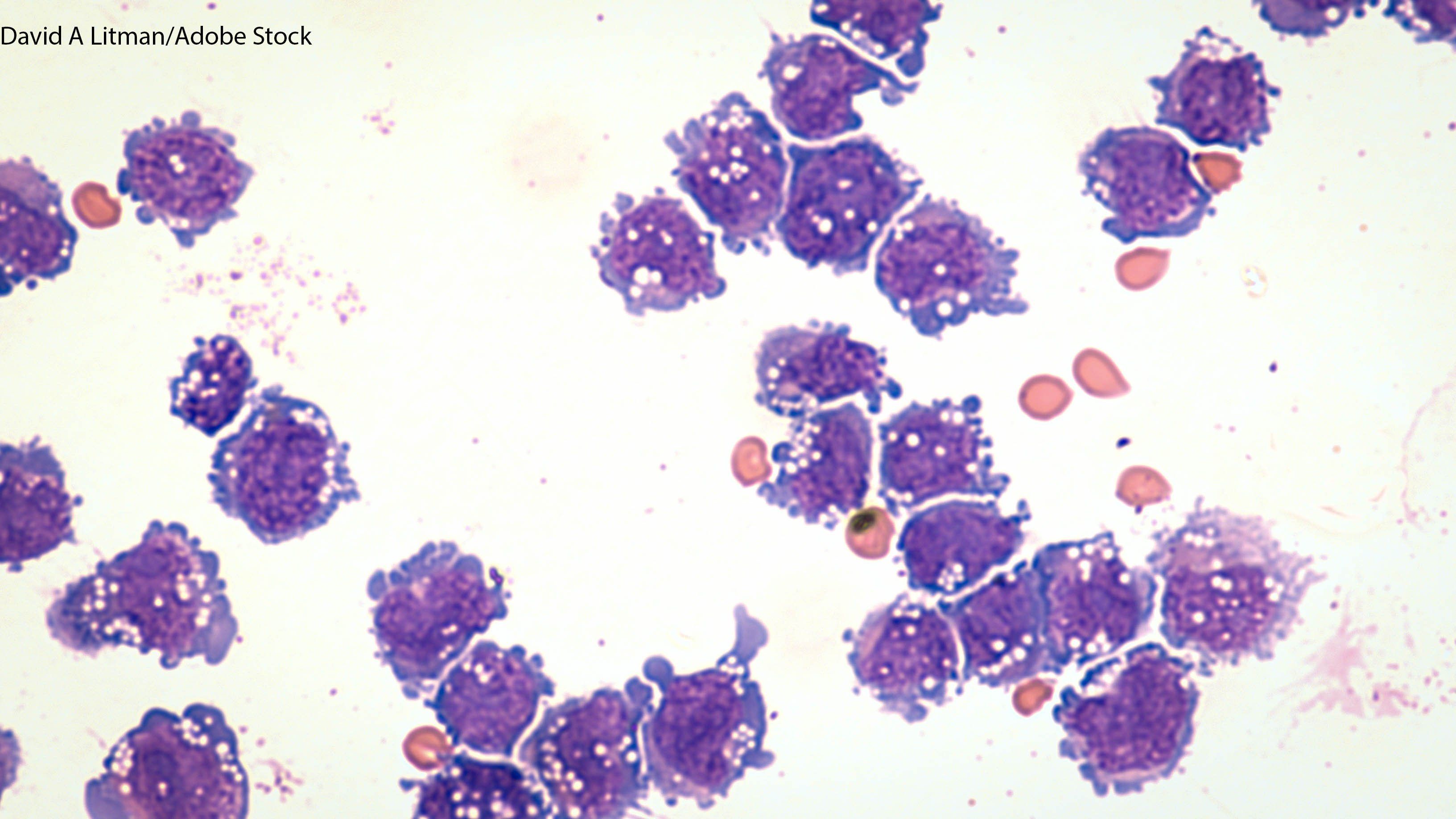
The submission to the FDA for the investigational CAR T-cell therapy is based on data from the phase II ZUMA-II trial for the treatment of adult patients with relapsed/refractory mantle cell lymphoma.

The associate professor at The Ohio State University Comprehensive Cancer Center discussed the implications of her analysis of the CAR T-cell therapy tisagenlecleucel for patients with diffuse large B-cell lymphoma at the ASH Annual Meeting & Exposition.


The Swedish Cancer Center expert discussed the addition of polatuzumab vedotin to a bendamustine-rituximab regimen at the ASH Annual Meeting & Exposition.

Andre H. Goy, MD, MS, from Hackensack University Medical Center, discussed ways to address the extension of survival for patients with DLBCL who achieved a complete remission at the ASH Annual Meeting & Exposition.
The ZUMA-II trial suggested that patients with relapsed/refractory mantle cell lymphoma resistant to prior therapies may benefit from the autologous anti-CD19 CAR T-cell therapy.

Matthew S. Davids, MD, MMSc, discussed his phase I/II study of duvalisib combined with venetoclax for patients with relapsed/refractory CLL and SLL at the ASH Annual Meeting & Exposition.
The combination of ibrutinib and rituximab demonstrated superior progression-free survival in older patients with previously untreated chronic lymphocytic leukemia.
The triplet regimen consisting of acalabrutinib, venetoclax, and obinutuzumab appeared highly active as frontline therapy for patients with chronic lymphocytic leukemia.

Lisocabtagene maraleucel induced high response rates in patients with aggressive relapsed/refractory large B-cell lymphoma.
Treatment with lenalidomide and rituximab improved progression-free survival, compared with placebo in patients ≥70 years old with indolent non-Hodgkin lymphoma.
The combination use of polatuzumab-vedotin, obinutuzumab, and lenalidomide showed high complete response rates in patients with relapsed/refractory follicular lymphoma.
Acalabrutinib alone and in combination with obinutuzumab significantly improved progression-free survival, compared with obinutuzumab plus chlorambucil in treatment-naïve patients with chronic lymphocytic leukemia
First-line treatment with ibrutinib plus venetoclax provided high rates of undetectable minimal residual disease in peripheral blood and bone marrow of patients with chronic lymphocytic leukemia.
Mosunetuzumab generated durable responses in patients with highly refractory non-Hodgkin lymphomas.
The CAR T-cell therapy axicabtagene ciloleucel induced a median overall survival of 25.8 months for patients with refractory large B-cell lymphoma.

The FDA granted a breakthrough therapy designation to abatacept for the prevention of moderate to severe acute graft-versus-host disease in hematopoietic stem cell transplants from unrelated donors.
Research on chimeric antigen receptor T-cell therapy to be presented at the ASH Annual Meeting & Exposition is set to address drawbacks associated with treatment.
MorphoSys announced that their ongoing phase III B-MIND study of tafasitamab passed the interim analysis for futility in patients with relapsed or refractory diffuse large B-cell lymphoma.