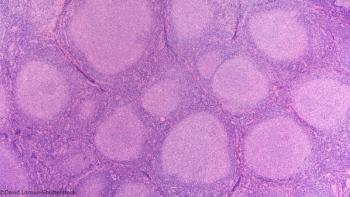
Despite improved outcomes seen in the rituximab era, the leading cause of death in patients with follicular lymphoma during the first decade remains lymphoma.

Your AI-Trained Oncology Knowledge Connection!


Despite improved outcomes seen in the rituximab era, the leading cause of death in patients with follicular lymphoma during the first decade remains lymphoma.
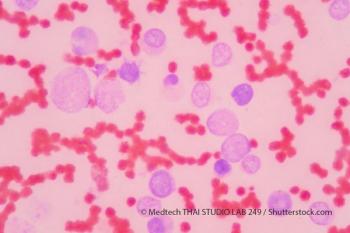
The US Food and Drug Administration recently approved a new agent for treatment of adult patients with relapsed or refractory acute myeloid leukemia.
The results of a follow-up analysis to the phase III MAVORIC study were presented at the ASH 2018 Annual Meeting & Exposition.
In the single-arm, multicenter, phase II part of the CAVALLI trial, the researchers analyzed the efficacy of 800-mg venetoclax plus R-CHOP in all first-line DLBCL patients.
Research presented at ASH 2018 examined whether checkpoint blockade therapy sensitizes patients with relapsed/refractory NHL to consequent treatment.
A new study has identified a clinically and biologically distinct subgroup of diffuse large B-cell lymphoma tumors.
A study looked at whether survivorship care plans were able to enhance patients' knowledge and adherence to physician recommendations.
Researchers studied clonal hematopoiesis of indeterminate potential (CHIP) as a way to assess risk for increased mortality in lymphoma patients.

Researchers developed an enhanced genomic model combining clinical factors and select gene mutations in order to better predict survival in patients with DLBCL who were administered first-line R-CHOP.

A study investigates whether R-CHOP is a viable first-line treatment for patients with advanced follicular lymphoma.
A phase III study looks at the efficacy and safety of rituximab biosimilar CT-P10 in patients with follicular lymphoma.
Researchers discovered clinical and experimental evidence that calls into question the widely held notion that LSCs preferentially outlive chemotherapy.
The ground-breaking study, led by the Leukemia & Lymphoma Society, is evaluating several novel targeted therapies for acute myeloid leukemia.

Cancer patients in England will receive a new game-changing therapy treatment under the first negotiated deal of its kind struck in Europe.

An inflammation marker was associated with several unfavorable characteristics in diffuse large B-cell lymphoma.

New research evaluated whether symptomatic indolent follicular lymphoma requires new long-term therapy after first-line rituximab without chemotherapy.

Evaluating the prognostic value of PET-CT responses after first-line immunochemotherapy in follicular lymphoma.
The research, published in JAMA Oncology, evaluates survival outcomes of a second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion.
A study in The Lancet Haematology suggests this combination could become first-line for elderly or unhealthy patients with newly diagnosed AML.
A study shows undergoing treatment for DLBCL at a clinic with more experience was linked with important clinical outcomes.

Plasma fibrinogen tests should be encouraged routinely in clinical practice to clarify its predictive benefit, according to a study.

Dr. Andrew Zelenetz spoke with Cancer Network about the importance of determining the cell of origin in patients with DLBCL.
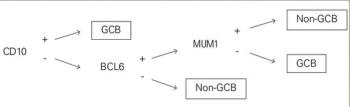
In this article, we review the methods of determining cell of origin (COO); use of COO in clinical practice; clinical trials in DLBCL according to COO; and future directions of tailoring treatment, including alternate categorization of genetic subtypes or clusters in DLBCL.

A study shows expanding access to care through insurance has the potential to improve outcomes in adults with follicular lymphoma.

A study shows pretreatment ctDNA levels and molecular responses are independently prognostic of outcomes in lymphomas.