
The agent is now the first and only approved CAR T-cell therapy to treat adult patients with relapsed or refractory mantle cell lymphoma.

Your AI-Trained Oncology Knowledge Connection!


The agent is now the first and only approved CAR T-cell therapy to treat adult patients with relapsed or refractory mantle cell lymphoma.
Researchers were able to establish possible mechanisms that may contribute to racial differences in incidence, patterns of presentation, and survival in patients with DLBCL with African ancestry.
Nirav Niranjan Shah, MD, discussed the use of autologous transplantation in patients with relapsed, chemosensitive DLBCL after the introduction of CAR T-cells.
The expert in hematology discussed what patients should know about the study, which evaluated the use of axicabtagene ciloleucel (axi-cel) in patients with relapsed or refractory indolent non-Hodgkin lymphoma.
The expert in hematology highlighted the toxicities reported in this trial and explained the differences observed between the 2 drugs studied.

Researchers found that dense allele-specific DNA methylation (ASM) mapping in normal samples plus cancer samples reveals possible candidate regulatory sequence polymorphisms (rSNPS) that are difficult to find by other approaches.

The FDA accepted and granted priority review to a supplemental biologics license application for pembrolizumab as monotherapy for the treatment of adult patients with relapsed or refractory classical Hodgkin lymphoma.
The FDA lifted the partial clinical hold on the on the pivotal phase 2 trial of camidanlumab tesirine (Cami) – designed to evaluate the antibody drug conjugate in patients with relapsed or refractory Hodgkin lymphoma.
Nirav Niranjan Shah, MD, discussed the study of patients with relapsed diffuse large B-cell lymphoma achieving only a PET/CT positive partial remission and what it means for this patient population.
Results from the phase 2 ZUMA-5 study indicated that axicabtagene ciloleucel (axi-cel) may be a promising approach for treating this patient population.
The expert in hematology discussed the next steps for the trial and what the goals are for the use of pembrolizumab in this patient population moving forward.
The study evaluated the use of pembrolizumab compared with brentuximab vedotin in patients with relapsed or refractory classic Hodgkin Lymphoma.

The FDA’s decision was based on a quality assessment of a new good manufacturing practice (GMP)-certified batch that was successfully manufactured for the lacutamab clinical development program.

The FDA approved oral selinexor for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma after at least 2 lines of systemic therapy.

The FDA approved tazemetostat for adult patients with relapsed or refractory FL whose tumors are positive for an EZH2 mutation as detected by an FDA-approved test and who have received at least 2 prior systemic therapies, as well as for adult patients with relapsed or refractory FL who have no satisfactory alternative treatment options.
The study assessed the use of axicabtagene ciloleucel in patients with relapsed or refractory indolent non-Hodgkin lymphoma.
The expert in hematology suggested that these findings represent a clinically meaningful benefit for patients that received pembrolizumab in the study, compared to those who received brentuximab vedotin.

Stephen Schuster, MD, talked about the benefits of conducting routine visits remotely during the COVID-19 pandemic, allowing doctors to see and treat more patients safely and efficiently.
Stephen Schuster, MD, discussed how they are prioritizing patients with more aggressive lymphomas, with emphasis on tumor volume and serum LDH levels during the COVID-19 pandemic.

The lymphoma and myeloma expert indicated that one of the key ways to address these disparities in lymphoma and myeloma is to improve minority and rural accrual in clinical trials.
Stephen Schuster, MD, explained how Penn Medicine is utilizing at-home treatments, which will continue after the pandemic, to maximize safety and reduce hospital traffic during the COVID-19 pandemic.
Stephen Schuster, MD, of Penn Medicine discussed testing for COVID-19, telemedicine and how they are adjusting their treatment of patients with aggressive lymphomas during the pandemic.
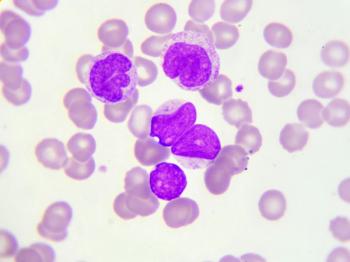
Test your diagnostic knowledge with this month's Image IQ.
Researchers found that 2 studied strategies, which combined nivolumab and doxorubicin, vinblastine, and dacarbazine, are feasible, highly effective, and result in excellent 12-month progression-free survival.
SGX301 is being evaluated for the treatment of patients with early-stage cutaneous T-cell lymphoma in the pivotal phase III FLASH study, which demonstrated that continued treatment twice weekly for 12 weeks increased the positive response rate.