Multiple Myeloma
Latest News

Latest Videos

CME Content
More News
High rates of durable complete responses are reported at the 2021 ASCO Annual Meeting with ciltacabtagene autoleucel in patients with relapsed or refractory multiple myeloma.

The CAR T-cell product idecabtagene vicleucel in patients with heavily pretreated multiple myeloma continued to show benefit of therapy at a median follow-up of 24.8 months.
Based on data from a phase 1 trial, the FDA granted breakthrough therapy designation to teclistamab for certain patients with pretreated multiple myeloma.
Based on data from the CARTITUDE-1, the BCMA-targeting CAR T-cell therapy ciltacabtagene autoleucel moves forward towards regulatory approval in multiple myeloma.

Recently announced phase 3 data show similar progression-free survival results between melflufen and pomalidomide, the most used medicine for patients with relapsed or refractory multiple myeloma.

CancerNetwork®’s podcast dives into an article focused on treatment options for older, transplant-ineligible patients with multiple myeloma.

Kathryn Maples, PharmD, BCOP, discusses real-world implications of belantamab mafodotin in patients with pretreated multiple myeloma.

A clinical pharmacy specialist discusses what’s needed to get the REMS program set up and touches on reimbursement considerations for the administration of belantamab mafodotin in patients with multiple myeloma.

A clinical pharmacy specialist details her experience with the REMS program for administration of belantamab mafodotin in patients with multiple myeloma.

Motixafortide Reaches Primary End Point of Improved Stem Cell Mobilization in Multiple Myeloma Trial
Statistical significance was reached across all end points of the phase 3 GENESIS trial investigating granulocyte colony stimulating factor plus either motixafortide or placebo in patients with multiple myeloma receiving autologous bone marrow transplantation.
Adding daratumumab led to at least a very good partial responses in all 41 patients receiving weekly carfilzomib, lenalidomide, and dexamethasone, and complete responses in all but 2 patients.
Dr Sagar Lonial, MD, FACP, shares his thoughts on the future of targeted therapies for patients with multiple myeloma.

Sagar Lonial, MD, FACP, provides insight on the potential role of BCMA-targeted therapies for newly diagnosed patients with multiple myeloma.

A leading myeloma expert reviews important data that led to the approval of belantamab mafodotin for heavily pretreated patients with relapsed/refractory multiple myeloma.

Dr Sagar Lonial, MD, FACP, comments on BCMA as a potential therapeutic target for multiple myeloma.

Sagar Lonial, MD, FACP, reviews targeted therapy options, and how they differ from other therapeutic approaches for relapsed/refractory myeloma.

A multiple myeloma expert introduces and defines relapsed/refractory myeloma and discusses typical treatment strategies.

Data on the use of ixazomib plus lenalidomide/dexamethasone were similar to results from the pivotal TOURMALINE-MM1 trial, suggesting patients with multiple myeloma in routine practice could achieve similar outcomes to patients treated on clinical trials.
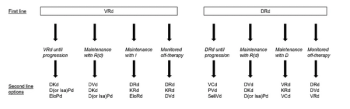
Radowan A. Elnair, MD, and Sarah A. Holstein, MD, PhD, review literature regarding progress in the management of the transplant-ineligible multiple myeloma in an article published in the journal ONCOLOGY®.
A higher mutational load detected by whole-genome sequencing in patients with multiple myeloma precursor conditions was likely predictive of subsequent disease progression.

The FDA approved the addition of isatuximab to the combination of carfilzomib and dexamethasone to treat adult patients with relapsed or refractory multiple myeloma who have received 1 to 3 prior lines of therapy.

The approval of idecabtagene vicleucel was supported by results from the phase 2 KarMMa trial, which evaluated the safety and efficacy of idecabtagene vicleucel (ide-cel) in patients who had received at least 3 prior regimens and were refractory to their last regimen per IMWG criteria.
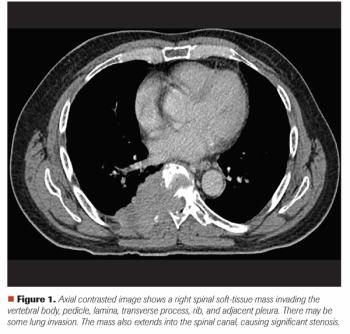
Mehmet S. Copur, MD, and colleagues examine the case of a 65-year-old who presented with back pain and a large T8 spinal mass, leading to a diagnosis of multiple myeloma with spinal cord compromise.

Data from the POLLUX and CASTOR trials both found higher sustained MRD-negativity rates for daratumumab combination regimens compared with the standard-of-care treatments.

“The importance of diverse representation cannot be underscored enough and is critical to ensure that safe and effective products are available to the [United States] patient population,” wrote the study authors, who were led by Nicole Gormley, MD.