
CancerNetwork®’s podcast features an interview with the chairman and CEO of Enveric Biosciences, David Johnson, to discuss the latest in their development of endocannabinoid products.

Your AI-Trained Oncology Knowledge Connection!


CancerNetwork®’s podcast features an interview with the chairman and CEO of Enveric Biosciences, David Johnson, to discuss the latest in their development of endocannabinoid products.
Take a look back at some of the important news and notes from last week in the world of oncology, featuring news from the FDA and articles about prostate cancer, ovarian cancer, and more.

Marron discussed the primary objectives and early data from a phase 1 trial presented at the virtual AACR Annual Meeting regarding the neoantigen peptide vaccine, PGV-001.

CancerNetwork® reports on updates in prevention and earlier diagnosis of colorectal, breast, and other cancers.

A presentation from the AACR Virtual Meeting: COVID-19 and Cancer detailed a literature review of natural therapeutics and remedies to combat the SARS CoV-2 virus.

Take a look back at some of the important news and notes from last week in the world of oncology, featuring articles about breast cancer, prostate cancer, and more.

Data investigating the predictive capabilities of high tumor mutation burden found it to be predictive of response to immune checkpoint blockade in some, but not all, types of cancers.
The American Cancer Society Cancer Action Network partnered with the National Comprehensive Cancer Network and the National Minority Quality Forum to produce recommendations addressing issues regarding health equity and disparities among minority patients with cancer.

Take a look back at some of the important news and notes from last week in the world of oncology, featuring news about prostate cancer, breast cancer, precision medicine, and gynecologic cancers.

Based on results of a cohort study that examined randomized clinical trials in 3 times periods, it is evident that efficacy measures and funding sources have dramatically changed over time.
CancerNetwork® spoke with Tzvia Bader about barriers between patients and appropriate clinical trials and how to combat these issues.

Take a look back at some of the important news and notes from last week in the world of oncology, featuring news about breast cancer, prostate cancer, molecular profiling, ovarian cancer, and more.

Compugen announced updated data surrounding the investigational therapeutic antibody, COM701, as monotherapy and in combination with nivolumab.
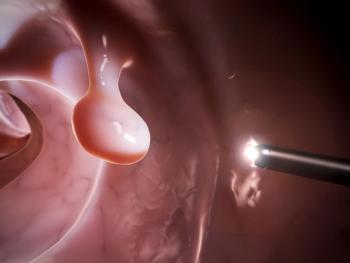
A novel imaging technology was cleared by the FDA to detect polyps with neoplastic potential during conduct of colonoscopy that aligns with pathological assessment with greater than 90% predictive ability.
Recently reported results from the ongoing phase 1 TUNINTIL trial of the oncolytic virus TILT-123 show safety of this approach.

Take a look back at some of the important news and notes from last week in the world of oncology, featuring news about breast cancer, prostate cancer, and urothelial cancer.

ONCOLOGY co–Editor-in-Chief Julie M. Vose, MD, MBA, details how underrepresentation of racial and ethnic minorities in clinical trials negatively impacts the medical community and calls for greater inclusion by removing some barriers to participation.
CancerNetwork® sat down with Trevor J. Pugh, PhD, to discuss a novel protocol for detecting mismatch repair deficiency.

Indications for pembrolizumab, nivolumab, and atezolizumab are set for examination under the FDA’s Oncologic Drugs Advisory Committee for indications granted accelerated approval in breast, urothelial, gastric, and liver cancers.
The liquid biopsy assay RaDaR has been given a breakthrough device designation for use in the detection of minimal residual disease in early-stage cancers.

Take a look back at some of the important news and notes from last week in the world of oncology, featuring news about prostate cancer, breast cancer, lung cancer, and more.

Schilsky joined CancerNetwork®’s podcast to discuss ASCO’s Advance of the Year, the top priorities moving forward, and the COVID-19 pandemic.
Giorgio Trinchieri, MD, of the National Cancer Institute’s Center for Cancer Research joined CancerNetwork® to discuss enhancing the gut microbiome by way of fecal transplant for better immunotherapy responses.
The medical oncologist and physician scientist based at the University of Texas MD Anderson Cancer Center explained recommendations for SARS-CoV-2 vaccination in patients participating in phase 1 oncology clinical trials.

Investigators suggest that results of a study may help to identify concerns of individuals undergoing panel testing and ways to support their needs.