
Immune checkpoint inhibitors have demonstrated impressive activity in patients with CRC and other solid tumors that are dMMR. Researchers explore the data.

Your AI-Trained Oncology Knowledge Connection!



Immune checkpoint inhibitors have demonstrated impressive activity in patients with CRC and other solid tumors that are dMMR. Researchers explore the data.

This year has seen many advances in immunotherapy. Here is a look at some of the most significant research.
The VOLFI trial enrolled 105 patients with RAS wild-type metastatic colorectal cancer.
Combination therapy with nivolumab and bevacizumab appeared to have clinical activity in ovarian cancer patients with platinum-sensitive or platinum-resistant disease.
A review of recent advances in the noninvasive imaging of immunotherapeutic targets.
Over that time, the number of active drugs in development has grown by more than 90%.
Age disparities in oncology trials are continuing, despite opposition to this issue.
While ibrutinib plus rituximab may improve progression-free survival in chronic lymphocytic leukemia, it is a costly option for some patients.
A total of 30 patients were enrolled in an expansion cohort for relapsed/refractory primary mediastinal B-cell lymphoma and received nivolumab and brentuximab vedotin.
Prostate cancer is the most frequent cancer found in American men, and the second leading cause of cancer death in this population. Currently, scores of reputable clinical trials are underway and recruiting patients.
Atezolizumab is an immune-checkpoint inhibitor, which has been approved by the US Food and Drug Administration (FDA) in various combinations for a number of cancer types in recent years.

Alan N. Peiris, MD spoke with ONCOLOGY about guidelines and strategies for treating patients who have had thyroid cancer.
The increasing utilization of these immune checkpoint inhibitors has presented new of immune-related adverse events that have proven to be extremely challenging to manage.
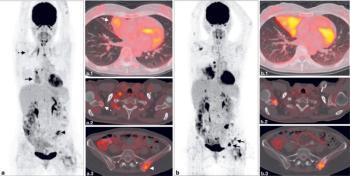
What is the best treatment option after progression to chemotherapy/immunotherapy (chemo/IO) as first-line treatment for PD-L1–positive lung adenocarcinoma with no sensitizing mutations?
The US Food and Drug Administration recently approved gilteritinib (Xospata), in addition to final trial data from the phase 3 ADMIRAL trial, making it the new standard of care for acute myeloid leukemia.
Brain metastasis is common in breast cancer and often has a poor prognosis, but there are several ways to manage brain metastasis in breast cancer including focal therapies as well as systemic options for specific populations, including emerging and novel therapies.

Accuracy of current guidelines for genetic testing of breast cancer patients has been recently challenged by a pair of studies suggesting that these guidelines may miss as many patients with pathogenic variants (or genetic mutations) as they may catch.

ONCOLOGY discussed therapy options, including chimeric antigen receptor (CAR)-T-cell therapies for pediatric acute lymphoblastic leukemia (ALL), with Susan R. Rheingold, MD, Medical Director of the Oncology Outpatient Clinic and attending physician with the Cancer Center at Children’s Hospital of Philadelphia.
A new meta-analysis compared survival in patients with advanced non–small cell lung cancer who received immunotherapy vs those who received chemotherapy.

Despite the toll chemotherapy can take on the body, experts say it's possible for athletes to exercise while undergoing treatment for cancer.

Among 598 trials published between 2003 and 2018, only 107 had a female corresponding author, though women made up anywhere from 27% to 39% of academic oncologists during this time period.
Negative trial findings suggest immune checkpoint inhibitors may not be the best type of immunotherapy to treat pancreatic cancer.
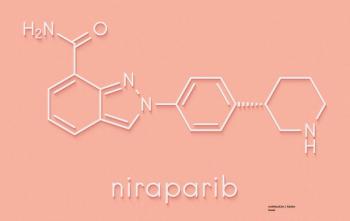
The TOPACIO trial tested the PARP inhibitor niraparib plus pembrolizumab in women with triple-negative breast cancer.
Nivolumab had limited efficacy against previously untreated brain metastases in patients with metastatic renal cell carcinoma, according to recent trial results.
A study in the Journal of the National Cancer Institute evaluated responses in men vs women to combination chemotherapy and a PD-1 or PD-L1 inhibitor.