
In a conversation during Hispanic Heritage Month, Ruben Mesa, MD, highlights factors that may contribute to disparities in clinical trial enrollment of Hispanic and Latinx patients with cancer and strategies that may encourage study participation.

Your AI-Trained Oncology Knowledge Connection!


In a conversation during Hispanic Heritage Month, Ruben Mesa, MD, highlights factors that may contribute to disparities in clinical trial enrollment of Hispanic and Latinx patients with cancer and strategies that may encourage study participation.
Colon Adjuvant Chemotherapy Based on Evaluation of Residual Disease (CIRCULATE-US): a prospective phase 2/3 trial of MRD-based adjuvant therapy for patients with early-stage colon cancer with intensified and deintensified adjuvant therapy approaches using ctDNA status as a surrogate for MRD status (NRG-GI008) (NCT05174169).

CancerNetwork® sat down with Ruben Mesa, MD, to discuss the disparities present in Hispanic and Latinx patients with cancer.
Ibrahim Halil Sahin, MD, and colleagues, explore, the CIRCULATE-US (NRG-GI008; NCT05174169) investigating postoperative ctDNA dynamics in early-stage colon cancer for treatment selection.
Michael Wang, MD, spoke about key data from the phase 3 SHINE trial which analyzed the use of first-line ibrutinib plus bendamustine and rituximab in older patients with mantle cell lymphoma.

Findings from a study examining the prognostic value of Nutritional Risk Index prior to hematopoietic stem cell transplant in patients with acute myeloid leukemia highlighted a need to consider other tools for assessing nutritional status among those undergoing transplant.

Patients with KRAS G12C–mutant non–small cell lung cancer may benefit from BBP-398 plus sotorasib, which received fast track designation from the FDA.
This article was written for and by CancerNetwork partner, the American Society for Transplantation and Cellular Therapy.

This article was written for and by CancerNetwork partner, the American Society for Transplantation and Cellular Therapy.
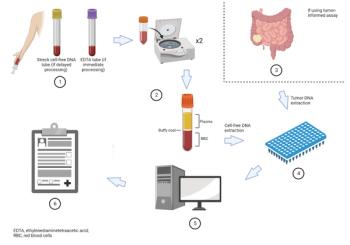
Ben Fangman, MD, and colleagues provide an overview of the use of circulating tumor DNA levels to detect minimal residual disease in colorectal cancer.

Ursula A. Matulonis, MD, spoke about the motivation behind the phase 3 SORAYA trial investigating mirvetuximab soravtansine in patients with folate receptor α–high platinum-resistant ovarian cancer.

Results from a retrospective cohort study indicated that vaginal brachytherapy yielded less radiation exposure to the female urethra in patients with endometrial cancer than external beam radiation therapy for those with colorectal cancer.

Findings from the phase 3 DREAMseq trial indicated that the best course of treatment for individuals diagnosed with advanced BRAF-mutated melanoma is first-line nivolumab/ipilimumab, with BRAF/MEK inhibitors used in later-line settings.

In an interview for Breast Cancer Awareness Month, Paolo Tarantino, MD, discusses the importance of multidisciplinary care for patients with breast cancer, the most exciting data presented this year, and prospects for future research.

Based on results from the phase 3 TROPiCS trial, the FDA has granted priority review to sacituzumab govitecan-hziy for unresectable locally advanced or metastatic hormone receptor–positive, HER2-negative breast cancer.

Jun J. Mao, MD, MSCE, spoke about updated guidelines from ASCO and SIO for pain management in patients with cancer.
Data from the phase 3 SUNLIGHT study highlighted the increased survival benefit of trifluridine/tipiracil plus bevacizumab compared with trifluridine/tipiracil alone for the treatment of metastatic colorectal cancer.
Co-editor-in-Chief Howard S. Hochster, MD, highlights exciting clinical trials that may set future standards for adjuvant therapy selection in colon cancer.
Kevin Kalinsky, MD, MS, spoke about future studies following results from the phase 2 MAINTAIN trial of ribociclib with or without endocrine therapy and after progression on a CDK4/6 inhibitor for patients with unresectable or metastatic hormone receptor–positive, HER2-negative breast cancer.

Patients with mantle cell lymphoma experienced higher relative risks of respiratory, blood, and infectious disease relative to the general population, regardless of treatment type.

After review by an independent data monitoring committee, the phase 2 ENVASARC trial, analyzing envafolimab monotherapy or in combination with ipilimumab for patients with undifferentiated pleomorphic sarcoma and myxofibrosarcoma, will proceed as planned.
Patients with metastatic colorectal cancer who were below a certain income level appear to be more susceptible to major financial hardship.

Joseph Mikhael, MD, spoke about the mechanism of action of bispecific antibody teclistamab relative to other agents in the space such monoclonal antibodies and CAR T-cell therapies in multiple myeloma.
Tafasitamab plus lenalidomide prolonged overall survival compared with systemic therapies in patients with relapsed/refractory diffuse large B-cell lymphoma, according to data from the retrospective RE-MIND2 study.

At 2022 IGCS, Lilian Gien, MD, spoke about a phase 2 trial which analyzed pembrolizumab plus epacadostat in patients with recurrent clear cell carcinoma of the ovary.

Following the 2022 American Society of Clinical Oncology Annual Meeting, experts in multiple myeloma discuss real-world evidence as it relates to the present-day treatment of patients.

The PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody was approved by the FDA as a companion diagnostic to help identify patients with HER2-low metastatic breast cancer who may be eligible to receive fam-trastuzumab deruxtecan-nxki.
The MAINTAIN trial was designed to assess endocrine therapy with or without ribociclib after progression on a CDK4/6 inhibitor in patients with unresectable or metastatic hormone receptor–positive, HER2-negative breast cancer.

Minimal residual disease negativity might be an important prognostic indicator in patients with newly diagnosed multiple myeloma, investigators say.

Findings of the phase 2 PERLA trial indicated that dostarlimab combined with chemotherapy achieved promising responses in patients with metastatic non-squamous non-small cell lung cancer.