
Patients with metastatic urothelial carcinoma who are susceptible to FGFR3 gene alterations may now receive erdafitinib, according to the FDA.

Your AI-Trained Oncology Knowledge Connection!


Patients with metastatic urothelial carcinoma who are susceptible to FGFR3 gene alterations may now receive erdafitinib, according to the FDA.

Data from the phase 3 LUNAR trial support the use of tumor treating fields in patients with non–small cell lung cancer.

Investigators are assessing avutometinib in combination with sotorasib as a treatment for those with KRAS G12C–mutated non–small cell lung cancer in the phase 1/2 RAMP-203 study.

Investigators indicate that ongoing efforts should focus on meeting the needs of adult cancer survivors with co-morbid substance use disorder, with an emphasis on prioritizing populations in which the disorder is highly present.

Overall survival also appears to improve with toripalimab compared with chemotherapy among patients with metastatic or advanced nasopharyngeal carcinoma.

In the overall population and in patients with a PD-L1 CPS of 5 or greater who received nivolumab plus chemotherapy, overall survival and progression-free survival improved when compared with chemotherapy alone.

Findings from a phase 1a/1b trial highlight that treatment with NX-5948 appears to be tolerable among patients with relapsed/refractory B-cell malignancies.

A group of experts discussed the approval process for teclistamab use in patients with multiple myeloma.
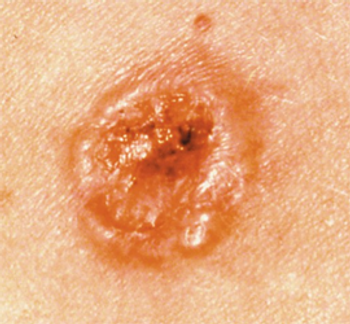
The DermaSensor device demonstrates a high rate of sensitivity in the detection of more than 200 types of skin cancers in a clinical study.

Results from an observational study found nivolumab plus chemotherapy helped enhance overall survival and progression-free survival for patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer, or esophageal adenocarcinoma.
Certain patients with salivary gland carcinoma and close surgical margins may safely be considered for observation, according to findings from a retrospective cohort study.

Developing novel regimens may continue to improve survival outcomes of patients with advanced cervical cancer following the FDA approval of pembrolizumab and chemoradiation, says Jyoti S. Mayadev, MD.
Data from a case control study highlight a decrease in the risk of breast cancer among those with increasing sex hormone binding globulin concentrations.

Investigators also report that patritumab deruxtecan is well tolerated in those with EGFR-mutant non–small cell lung cancer in the HERTHENA-Lung01 study.

Findings from a secondary analysis of a phase 3 trial support stereotactic radiosurgery as a standard of care for those with brain metastases, although whole-brain radiotherapy may yield more local and distant control.

An overview of NRG1 fusion–positive tumors was given by experts in the gastrointestinal and lung cancer space in a recent Frontline Forum.

Treatment with pembrolizumab plus chemoradiation appears to be well tolerated with no detriment to quality of life among those with advanced cervical cancer.

Investigators report favorable overall survival among patients with chronic lymphocytic leukemia who receive ibrutinib/venetoclax over FCR.

Ghayas C. Issa, MD, gave an all-encompassing review of current treatment options for acute myeloid leukemia and what to expect in the future.

Findings from a randomized trial highlight that treatment with mirtazapine may lead to an improvement in health-related quality of life among those with advanced non–small cell lung cancer and anorexia.

Adding adaptive radiation to chemotherapy may offer a novel approach to treating patients with advanced non–small cell lung cancer, according to Michael Steinberg, MD.

Treatment with imetelstat produces robust activity in patients with relapsed/refractory myelodysplastic syndrome regardless of ring sideroblasts in the phase 3 IMerge trial.
Investigators note that the phase 3 VESPER study’s data support the use of 6 neoadjuvant cycles of dose-dense MVAC vs 4 in muscle-invasive bladder cancer.

Adding pegylated L-asparaginase to consolidation chemotherapy demonstrates a modest decrease in minimal residual disease negativity among those with high-risk acute lymphoblastic leukemia.

Ciara Kelly, MBBCh, BAO, discusses personalized treatment approaches for patients with soft tissue sarcoma.

Sarah Donahue, MPH, NP, speaks to the importance of communicating potential adverse effects associated with treatments such as CDK4/6 inhibitors to patients with breast cancer.

Black male patients with breast cancer appear to experience worse survival outcomes compared with White patients when controlling for clinicopathological variables, according to Jason (Jincong) Q. Freeman, MPH, MS.

Thomas Hutson, DO, PharmD, meets with his patient Cesar Fuentes to discuss his diagnosis and treatment for renal cell carcinoma.
Patients with advanced ovarian cancer who received primary or interval cytoreduction surgery experienced higher rates of overall and progression-free survival.

Findings from the phase 2 JACKPOT8 study support golidocitinib as a potential treatment option for those with relapsed or refractory peripheral T-cell lymphoma.