
Shubham Pant, MD, highlights the importance of tailoring treatment in patients with pancreatic cancer by exploring biomarkers.

Your AI-Trained Oncology Knowledge Connection!


Shubham Pant, MD, highlights the importance of tailoring treatment in patients with pancreatic cancer by exploring biomarkers.

The 3-year follow-up update from the phase 2 ZUMA-2 trial showed continued responses for patients with relapsed/refractory mantle cell lymphoma treated with brexucabtagene autoleucel.

Rahiya Rehman, MD, and co-investigators, research the importance of poor follow-up and care for survivors of childhood cancer.

Independent factors such as the integrated clinical radiomics model and 18F-FDG PET/CT radiomics signature may be predictive of progression-free survival in breast cancer.

Patients with heavily pretreated multiple myeloma appeared to benefit from treatment with talquetamab and daratumumab plus hyaluronidase-fihj.

Biagio Ricciuti, MD, spoke about the 3-year follow-up and prolonged survival benefit in patients with non–small cell lung cancer who had very high PD-L1 expression of 90% or more.

This review article written by Meghana Kesireddy, MBBS and Matthew A. Lunning, MD, reviews management and treatment of relapsed/refractory large B-cell lymphoma.
Data from the SPOTLIGHT trial indicate agreement among trained PET readers was highly concordant with use of 18F-rhPSMA-7.3 imaging for recurrent prostate cancer.
Patients with treatment-naïve chronic lymphocytic leukemia who were treated with venetoclax and obinutuzumab with or without ibrutinib experienced a progression-free survival benefit compared with standard chemoimmunotherapy.

Richard Kim, MD, highlights future research efforts examining immunotherapy/chemotherapy combinations for patients with metastatic colorectal cancer.
Karen L. Reckamp, MD, spoke about the rationale behind the Lung-MAP S1800A substudy of pembrolizumab plus ramucirumab for patients with non–small cell lung cancer and the topline results from 2022 ASCO.
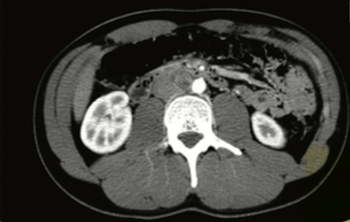
In this installment of Clinical Quandaries, Bendu Konneh, BS, and colleagues present a case of a 21-year-old male with a 4-month history of progressive swelling in the right testicle.

Despite women with multiple myeloma being more likely to have certain cytogenetic risk factors vs men, sex did not appear to impact efficacy.

Patients with uterine carcinosarcoma who underwent sentinel lymph node biopsy or systemic lymph node dissection did not experience a difference in progression-free survival or overall survival.
Tony S. Mok, MD, spoke about the use of capmatinib plus pembrolizumab in patients with previously untreated non–small cell lung cancer who were MET unselected and had PD-L1 expression of 50% or more.

Hope S. Rugo, MD, FASCO, spoke about future analyses of the phase 3 TROPiCS-02 trial that examined sacituzumab govitecan vs physicians’ choice chemotherapy in patients with hormone receptor–positive, HER2-negative advanced breast cancer.

“Be courageous: Your work will be recognized, and everything is about [the] work in the end.”

Based on results of the phase 3 KEYNOTE-091/PEARLS trial, the FDA has accepted a supplemental biologics license application for pembrolizumab for patients with stage IB, II, or IIIA non–small cell lung cancer after a complete resection.
Tom Thomas, MD, highlights how the stigma surrounding human papillomavirus–associated head and neck cancer is being unraveled through education and the importance of vaccination as a prevention strategy.
Based on results of the phase 3 RATIONALE-309 trial, the China National Medical Products Administration has approved tislelizumab plus chemotherapy for the first-line treatment of recurrent or metastatic nasopharyngeal cancer.

Richard Kim, MD, discussed key findings of the phase 1b KEYNOTE-651 trial, examining pembrolizumab plus standard chemotherapy in patients with microsatellite-stable or mismatch repair–proficient colorectal cancer.

The combination of quizartinib plus chemotherapy yielded better survival outcomes vs chemotherapy alone in the frontline setting of FLT3-ITD-positive acute myeloid leukemia.

An expansion cohort from the phase 2 EPCORE NHL-1 trial demonstrated strong efficacy of epcoritamab in patients with relapsed/refractory large B-cell lymphoma.

In this month's Letter to the Readers, ONCOLOGY co-editor-in-chief Julie M. Vose, MD, MDA, dives into the topic of survivorship care.

Shubham Pant, MD discusses key findings from a basket trial examining the use of erdafitinib in patients with gastrointestinal and other solid tumor malignancies.

Expert panelists focus on the treatment of metastatic urothelial cancer and discuss strategies and challenges for maintenance therapy.
Follow-up results of the phase 3 ASPEN trial comparing zanubrutinib with ibrutinib in patients with Waldenström macroglobulinemia were presented at 2022 ASCO.

Results of the phase 3 RATIONALE-309 trial demonstrate efficacy of tislelizumab added to chemotherapy for recurrent or metastatic nasopharyngeal carcinoma.

Treatment with the oral VEGFR2 inhibitor rivoceranib appears to induce responses in recurrent or metastatic adenoid cystic carcinoma.

During the 2022 American Society of Clinical Oncology Annual Meeting, Richard Kim, MD, discusses the rationale for the phase 1b KEYNOTE-651 trial examining pembrolizumab plus chemotherapy in metastatic colorectal cancer.